Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2009 |
Exam 2 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2009 |
Exam 2 |
![]()
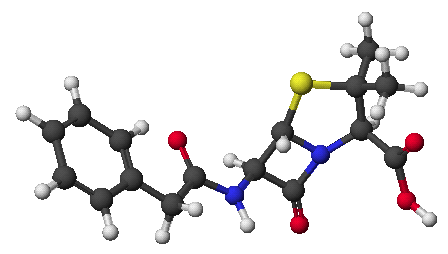
1. (15 points) Write complete names for each of the following:
a) 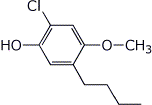
b) 
c) 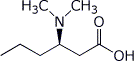
d) 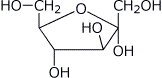
e) 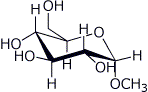
2. (15 points) Write complete structures for the following:
a) L-glucose (open-chain)
b) 2,3-dinitropyridine
c) p-hydroxybenzenediazonium fluoborate
d) beta-D-ribofuranose
e) beta-D-ribopyranose (Haworth form)
3. (15 points) Arrange the following in order by writing MOST and LEAST under the compounds with the highest and lowest values of the indicated property.
a) acidity
phenol / / aniline / / p-cyanophenol
b) basicity
pyrrole / / aniline / / diethylamine
c) reactivity towards nucleophilic substitution
2,4-dinitrofluorobenzene / / 2,4-dinitrochlorobenzene / / 2,3-dinitrochlorobenzene
d) number of secondary alcohol groups (specify the number)
D-glyceraldehyde / / D-fructose / / D-glucose
e) number of chirality centers (specify the number)
D-ribofuranose / / D-glucopyranose / / D-fructofuranose
4. (15 points) Complete each of the following reactions by adding the structure of the expected final product or products. Show stereochemistry if it is specific.
a) ![]()
b) 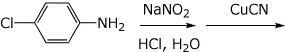
c) 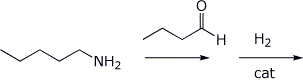
d) 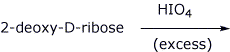
e) 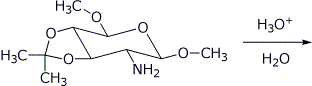
5. (20 points) Show a sequence of reactions that could be used to prepare the following.
a) m-chlorophenol, starting from benzene and at least once using a diazonium ion
b) 1-butene, starting from butane, using a Hoffman elimination
6. (10 points) D-Apiose (shown below) is an unusual branched sugar with a tertiary alcohol.
a) Write the structure for L-apiose.
b) When D-apiose forms a furanose ring, there are TWO new stereocenters created. Write structures for all four of the diastereomers that could be formed of D-apiofuranose.
7. (10 points) Refer to the disaccharide shown below.
a) On the structure above, identify each anomeric carbon with an asterisk (*) and indicate whether the structure is alpha or beta.
b) Describe the glycoside linkage between the two monosaccharides.
c) After complete hydrolysis, write structures (open-chain) for the two monosaccharides that would result. Describe each one in relation to D-glucose.