Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2009 |
Exam 1 Answer Key |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2009 |
Exam 1 Answer Key |
![]()
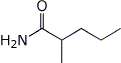
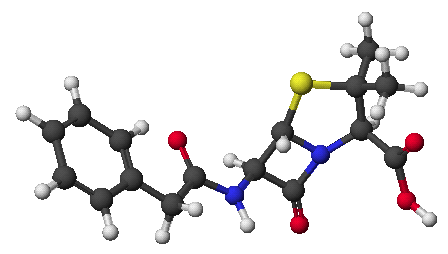
1. (15 points) Write complete names for each of the following:
a) ![]()
b) 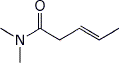
c) ![]()
d) ![]()
e) ![]()
2. (15 points) Write complete structures for the following:
a) benzyl p-phenylbenzoate
b) propanoic (R)-2-chloropropanoic anhydride
c) lithium m-nitrophenylacetate
d) diethyl dimethylmalonate
e) the enol of acetyl chloride
3. (15 points) Shown below are tetrahedral intermediates that could be in equilibrium with two different forms of carbonyl or carboxyl derivatives. Write balanced equilibrium reactions for each case (uncharged forms).
a) 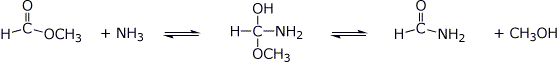
b) 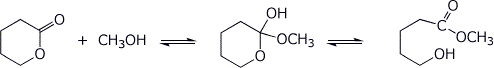
c) 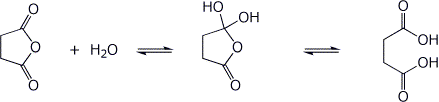
d) 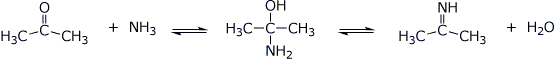
e) 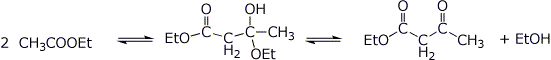
4. (15 points) Complete the following reactions, including stereochemistry if it is specific:
a) 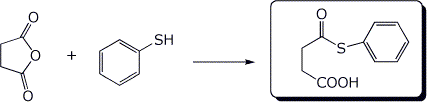
b) 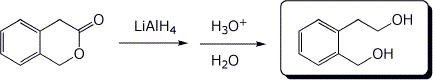
c) 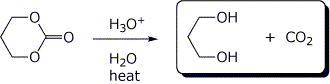
d) 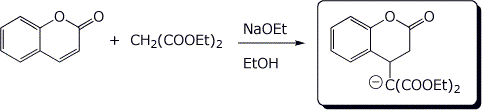
e) 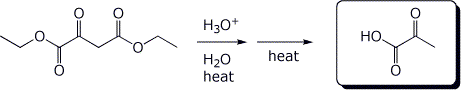
5. (15 points) Write a complete mechanism for the acid-catalyzed reaction below. Show all steps and all resonance forms for any intermediates involved. You may use H+ and -H+ to show proton transfer steps.
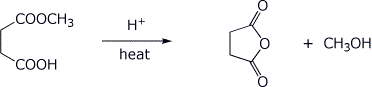
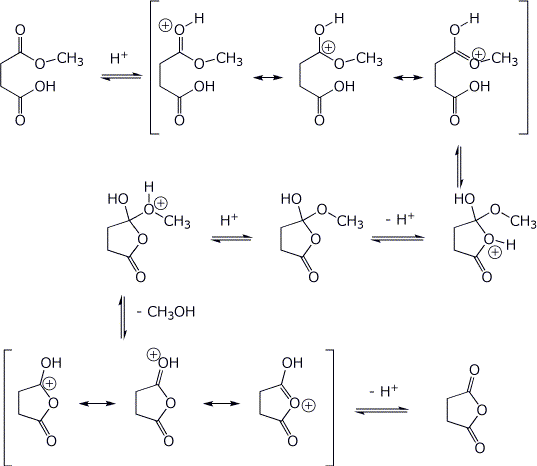
6. (15 points) Treatment of the ketoester below with base followed by an acid workup gives two products. Write complete mechanisms that show how each product could be formed. It is not necessary to write all resonance forms of intermediates.
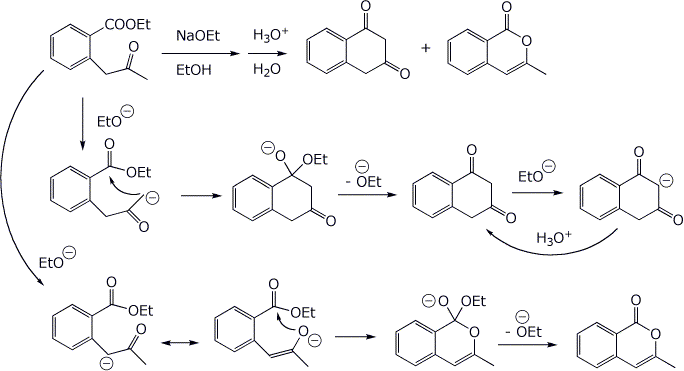
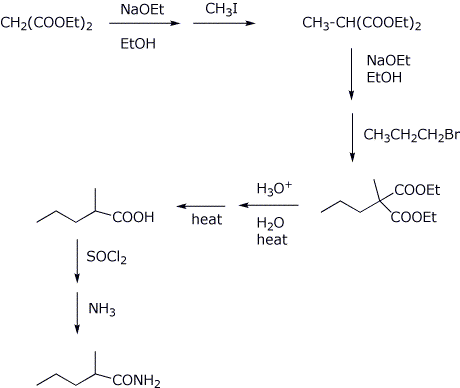
7. (10 points) Show how to synthesize the target compound below, using a malonic ester synthesis.