Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Quiz 28 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Quiz 28 |
![]()
1. (3 points) Write a complete structure for 2’-deoxyguanosine-3’-diphosphate, as it would appear at pH 7.
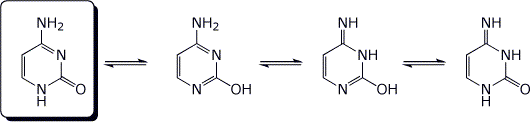
2. (4 points) Circle the most stable form of cytosine among the four tautomers below.
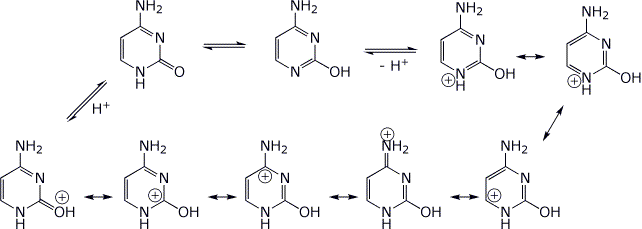
Conversion of the keto to the enol form (1 → 2) involves protonation of the keto form to create a cation, for which there are seven (7) resonance forms . Write them all.
3. (3 points) Show the structure of just the bases for A and T, including the H-bonding pattern between them.