Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Quiz 27 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Quiz 27 |
![]()
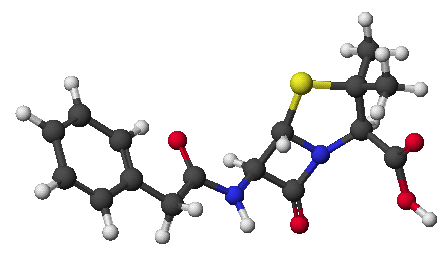
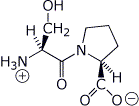
1. (2 points) Write the structure of the dipeptide Ser-Pro as it would be at pH = 7, including stereochemistry.
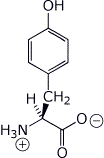
2. (2 points) Write the expected structures, including stereochemistry, of each amino acid as it would exist at its pI:
a) Tyr ( pI = 5.66 )
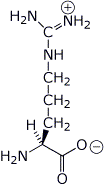
b) Arg ( pI = 10.76 )
3. (3 points) Write out the complete side chain structures to illustrate the following types of interactions:
a) H-bonding between Gln and Thr
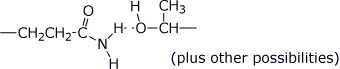
b) covalent bonding between Cys and Cys
![]()
c) Coulombic (charge) attraction between Lys and Asp
![]()
4. (3 points) Identify the unknown pentapeptide based on the following observations.
Complete hydrolysis releases Ala, Ile, Lys, Trp, Val .
Edman analysis
releases Ile .
Carboxypeptidase releases Ala .
Trypsin generates a dipeptide
(A) and a tripeptide (B).
Chymotrypsin generates a dipeptide (C) and
a tripeptide (D).
Both A and D are shown to have Ile at their N-terminus.
Ile - Lys - Trp - Val - Ala