Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Quiz 25 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Quiz 25 |
![]()
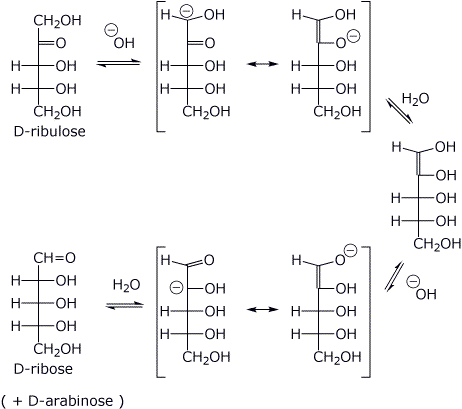
1. (2 points) The ketopentose shown below is D-ribulose.
Write
a clear structure for α-D-ribulofuranose.

2. (3 points) D-ribulose (see above) interconverts with D-ribose via an
enediol.
Write a complete mechanism for the interconversion using NaOH
as base catalyst.
Use open-chain structures and show all resonance forms.
3. (2 points) Show the products expected upon acid-catalyzed hydrolysis (dilute aqueous acid) from the following. Show sugars as open-chain forms.
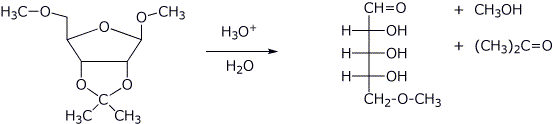
4. (3 points) Referring to the disaccharide shown below,
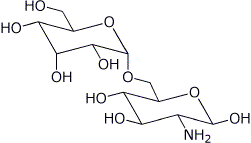
a) put * by each anomeric carbon and identify each as alpha or beta
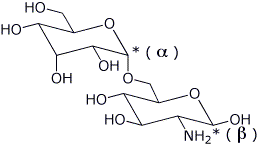
b) identify the glycoside linkage by position and stereochemistry
a1,6
c) describe the structure of each sugar unit relative to D-glucose
the left sugar is the C-3 epimer of D-glucose
the right sugar is D-glucose with a 2-amino group with the same stereochemistry