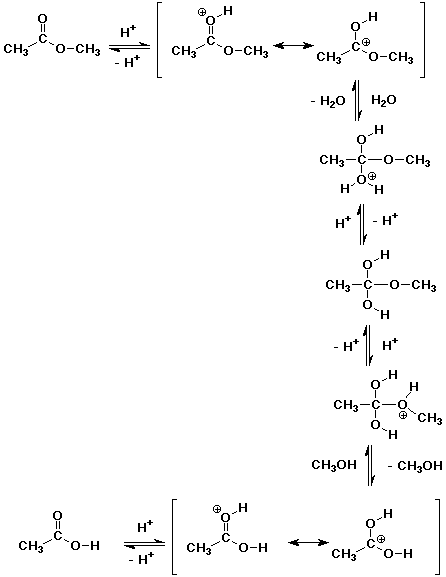|
|
Organic
Chemistry III
|
|
Professor
Carl C. Wamser
|
|
|
Chapter
19 Homework Answers
|

Carey, pages 855 - 861
Problems 19.13 - 27 , 31 - 34
1. Give IUPAC names for the following:
a) 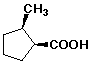
(1S,2R)-2-methylcyclopentanecarboxylic acid
Note - (cis) is correct but not specific enough to identify this enantiomer
b) 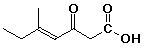
(E)-5-methyl-3-oxo-4-heptenoic acid
(trans) would also be acceptable
2. Write a complete mechanism for the acid-catalyzed hydrolysis of methyl
acetate.
The mechanism is the exact reverse of Fischer esterification.
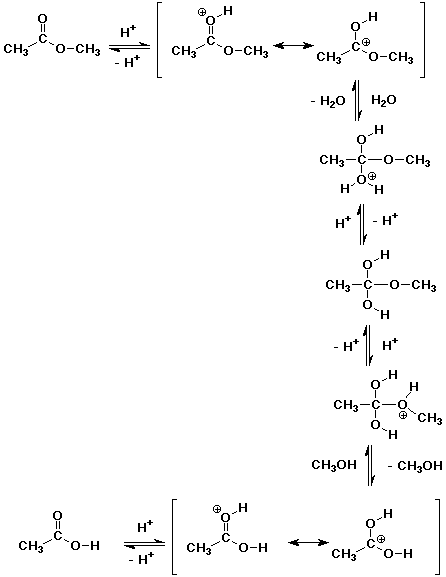
3. Show two different ways to prepare cyclohexanecarboxylic acid starting
with cyclohexane.
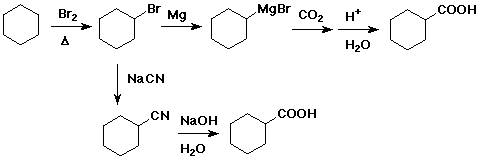
The upper pathway is likely to be better
because the SN2 displacement by
CN- on the 2° alkyl bromide is likely to
be slow and may be complicated by
some elimination.


![]()