Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Exam 3 Answer Key |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Exam 3 Answer Key |
![]()
1. (20 points) Write clear structures, with stereochemistry, of the following as they would appear at pH 7.
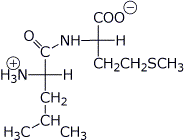
a) LeuMet
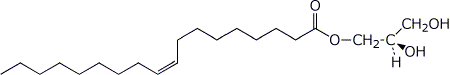
b) (R)-stereoisomer of the monoglyceride of oleic acid (C18 monounsaturated at ω-9)
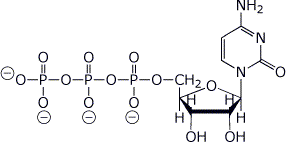
c) cytidine-5’-triphosphate
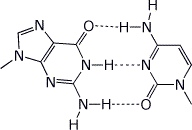
d) a GC base pair (just the bases, with the H-bonding)
2. (15 points) Complete the following reactions showing the structures of the expected major products, including stereochemistry:
a) BOC-Ala + DCC →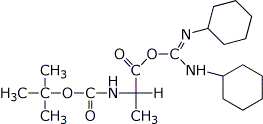
b) Val + CH3OH + HCl →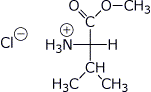
c) acetyl coA
+ glycine → ![]()
d) adenine + NaNO2, HCl, H2O →
e) phosphatidic acid of glyceryl dihexadecanoate + excess NaOH, H2O , heat →
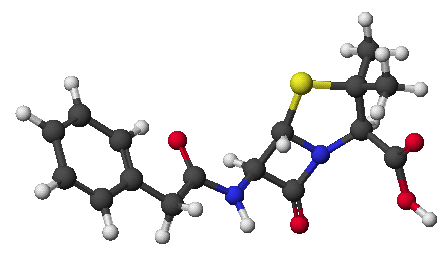
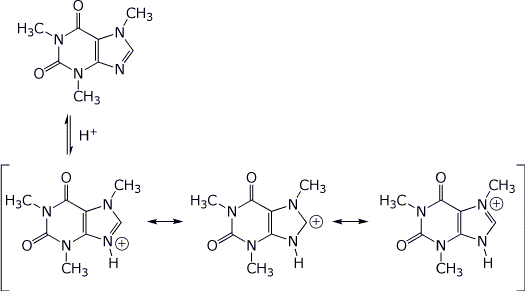
3. (15 points) The structure of caffeine is shown below. Rewrite it to show its fully aromatic resonance form.
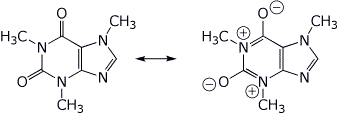
Which of the four nitrogens is expected to be the most basic? Show all three of the resonance forms for caffeine protonated at that nitrogen.
4. (10 points) Identify the unknown heptapeptide based on the observations described below.
Complete hydrolysis gives Arg, Gly, Lys,
Ser, Thr, and 2 Tyr .
Chymotrypsin cleavage gives A, B, and C .
Trypsin
cleavage gives D, E, and Ser .
A, B, and D are shown to be dipeptides.
Edman degradation on the original heptapeptide, C, or D, all give Thr
followed by Lys .
Thr Lys Tyr Gly Tyr Arg Ser
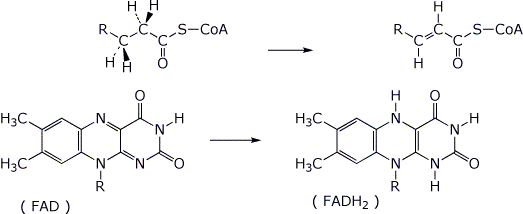
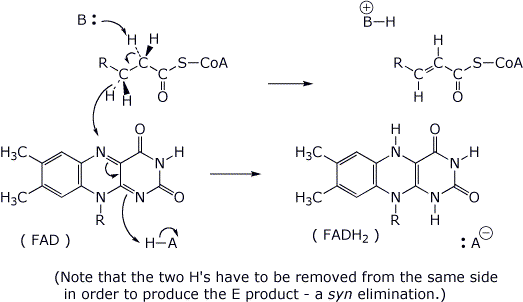
5. (15 points) FAD is a dinucleotide used in biological redox reactions. A general example is shown below. Add electron-pushing arrows to the reaction (on the left) to show how to arrive at the indicated products on the right. Also indicate (on the left) where a clever enzyme would place acid and base catalysis to enhance the reaction.
( R represents
the adenine dinucleotide portion of FAD, which is not involved in the
reaction. )
Take into account that the product is specifically E stereochemistry.
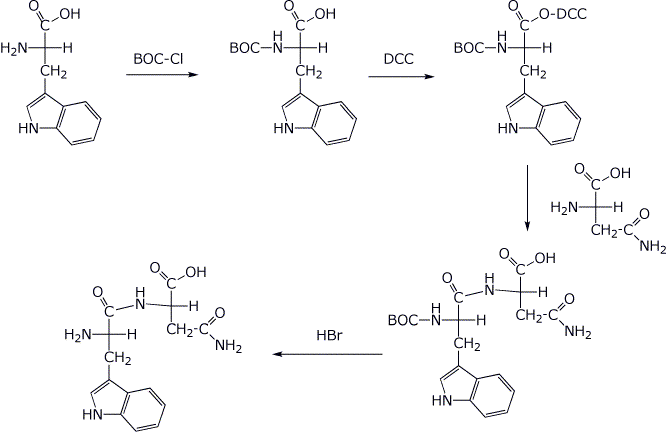
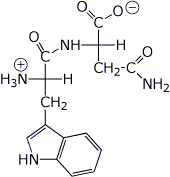
6. (25 points) Your project is to learn all about the dipeptide TrpAsn .
a) (4 points) Write the structure (with stereochemistry) of TrpAsn as it would appear at pH 7.
b) (4 points) Write all the expected products that would be formed from TrpAsn upon complete hydrolysis at pH 1 (stereochemistry optional).
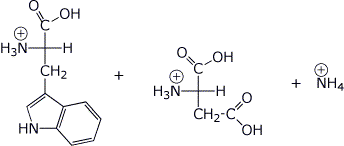
c) (4 points) What products would be expected (just the amino acid abbreviations are OK) upon treatment of TrpAsn with
i) Edman’s reagent ---> labeled Trp + Asn
ii) Chymotrypsin ---> Trp + Asn
iii) Trypsin ---> no reaction
iv) Carboxypeptidase ---> Trp + Asn
d) (4 points) What are all the possible m-RNA codons for TrpAsn?
UGG-AAU or UGG-AAC
e) (9 points) Show the steps in a possible synthesis of TrpAsn, starting with the individual amino acids plus BOC and DCC. You may abbreviate BOC and DCC.