Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Exam 2 Answer Key |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Exam 2 Answer Key |
![]()
1. (15 points) Give complete names for each of the following:
a) ![]()
b) ![]()
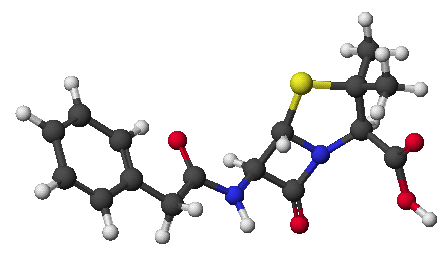
c) 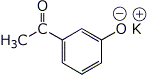
d) 
e) 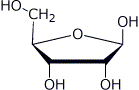
2. (15 points) Draw accurate structures for the following:
a) N-methyl-N-nitrosoaniline
b) 2,6-dibromobenzoquinone
c) L-glyceraldehyde
d) sucrose
e) chair form of O-methyl β-D-glucopyranoside
3. (15 points) Arrange the following in order by writing MOST and LEAST under the compounds with the highest and lowest values of the indicated property.
a) basicity
MOST / / / LEAST / / / MIDDLE
b) acidity
MOST / / / MIDDLE / / / LEAST
c) rate of nucleophilic substitution
LEAST / / / MIDDLE / / / MOST
d) number of chirality centers
MOST / / / MIDDLE / / / LEAST
e) number of moles of HIO4 consumed
MIDDLE / / / LEAST / / / MOST
4. (15 points) Complete each of the following reactions by adding the structure of the expected final product or products. Show stereochemistry if it is specific.
a) 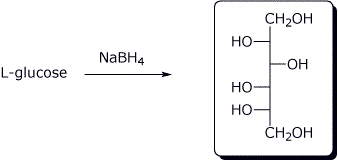
b) (show the products after each stage)
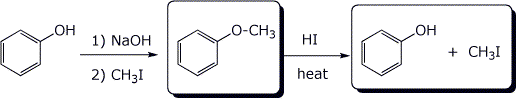
c) 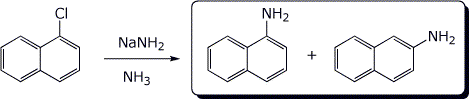
d) 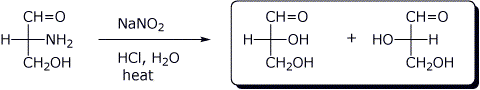
e) 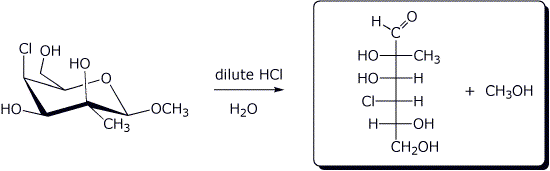
5. (10 points) Write a complete mechanism for the substitution reaction shown below. Show all resonance forms for the intermediate and circle the most stable resonance form.
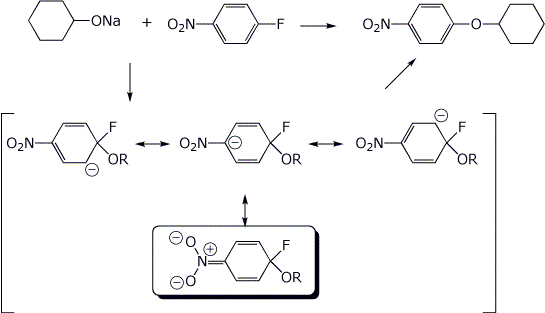
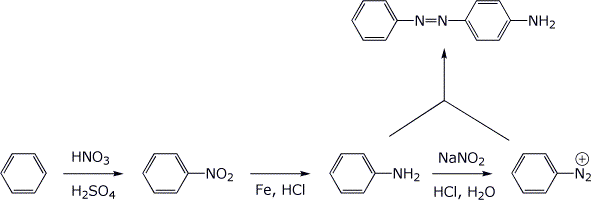
6. (10 points) Show a sequence of reactions that could be used to prepare the compound below, starting with benzene. Use diazonium ions.
7. (10 points) D-Mannose is the C-2 epimer of D-glucose. On treatment with acid and methanol, it forms several O-methyl glycosides. Write structures for the following:
a) β-furanoside
b) β-pyranoside (Haworth form)
c) β-pyranoside (chair form)
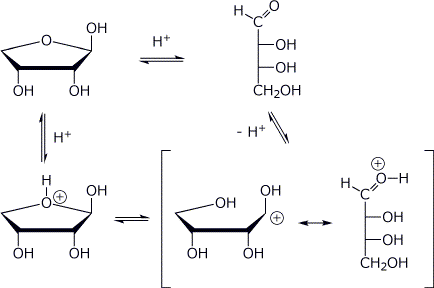
8. (10 points) Write a mechanism for the interconversion of the furanose to the open-chain form of D-erythrose (shown below). Show all steps and all resonance forms for any intermediates.