Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Exam 1 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Exam 1 |
![]()
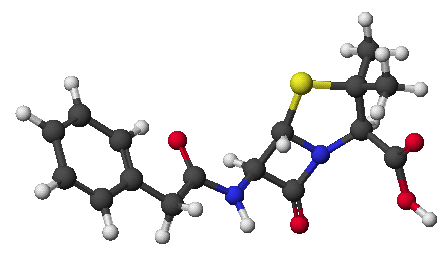
1. (15 points) Write complete names for each of the following:
a) 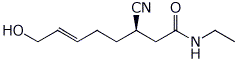
b) 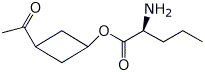
c) 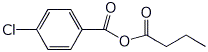
2. (15 points) Write complete structures for the following:
a) the neutral tetrahedral intermediate in the formation of ethyl benzoate from the acid chloride
b) the saponification products from methyl decanoate
c) tetramethylammonium trichloroacetate
d) the H-bonded enol of malonic ester
e) (R)-3-cyanohexanedioic anhydride
3. (15 points) Complete the following reactions, including stereochemistry if it is specific:
a) 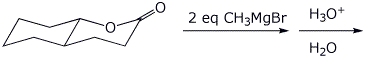
b) 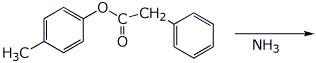
c) 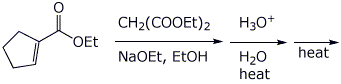
d) 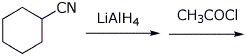
e) 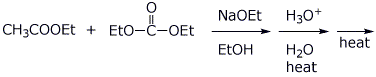
4. (20 points) Write a complete mechanism for the acid-catalyzed substitution reaction below.
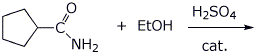
5. (20 points) Show how to synthesize each of the following. You may start with acetoacetic ester, malonic ester, benzene, and any alcohol of three carbons or fewer.
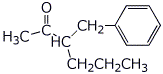
![]()
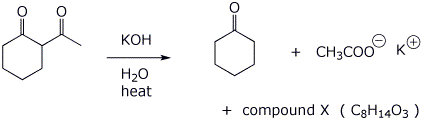
6. (15 points) Upon treatment with base, beta-diketones can be cleaved, as shown below. Write a complete mechanism to show how this occurs. Show all steps and all resonance forms for any intermediates involved.
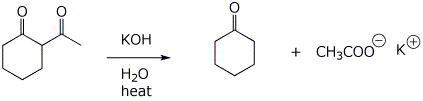
That reaction also produces another product, compound X, a ketocarboxylic acid. Write a comparable mechanism, explaining how this product arises.