Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Chapter 21 Notes |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2008 |
Chapter 21 Notes |
![]()
![]()
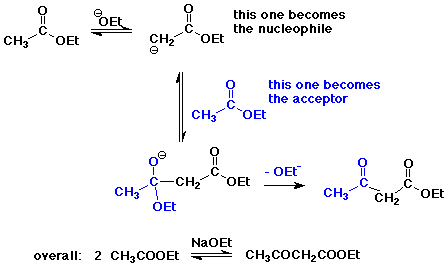
The Claisen Condensation
Decarboxylation of beta-ketoacids
Acetoacetic Ester and Malonic Ester Syntheses
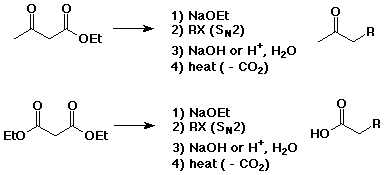
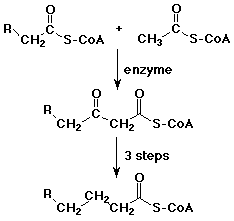
Biological Claisen Reactions