Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2007 |
Quiz 27 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2007 |
Quiz 27 |
![]()
1. (3 points) Show the structures of the individual amino acids that would be generated by the complete hydrolysis of the tripeptide Pro-Leu-Thr as they would exist at pH 1. Stereochemistry is not required.
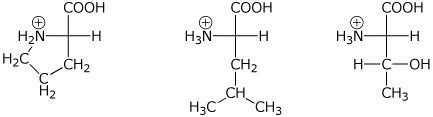
2. (4 points) Show the complete structure of the products that would be formed in the following reactions. Include specific stereochemistry.
a)
Cys + excess CH3COCl ---> 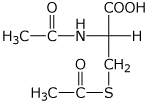
b) Ala + Ph-CH2-O-CO-Cl (Z) ---> 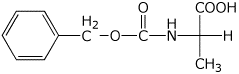
3. (3 points) Identify the unknown hexapeptide based on the following data.
Complete hydrolysis gives Arg, Ile, Phe, Ser, Trp, Val
N-terminal analysis gives Ile
Carboxypeptidase releases Phe
Chymotrypsin creates a dipeptide (A) and a tetrapeptide (B)
Trypsin creates two tripeptides (C and D)
Both A and C have Ile as the N-terminus, D has Val at the N-terminusIle - Trp - Arg - Val - Ser - Phe