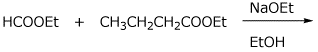Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2007 |
Quiz 21 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2007 |
Quiz 21 |
![]()
1. (2 points) Show the expected final product from the following reactions:
a) 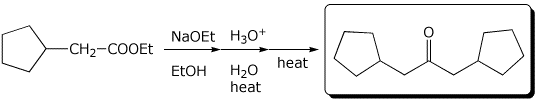
b) 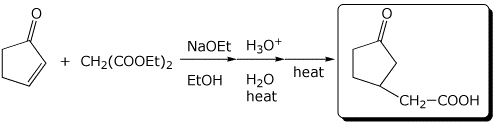
2. (2 points) Show how the compound below would be prepared via an acetoacetic ester synthesis.
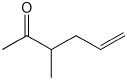
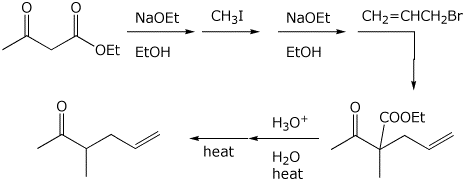
3. (2 points) Show the steps needed to accomplish the following conversion.
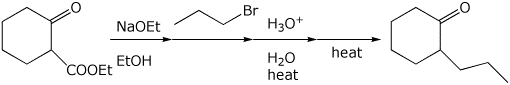
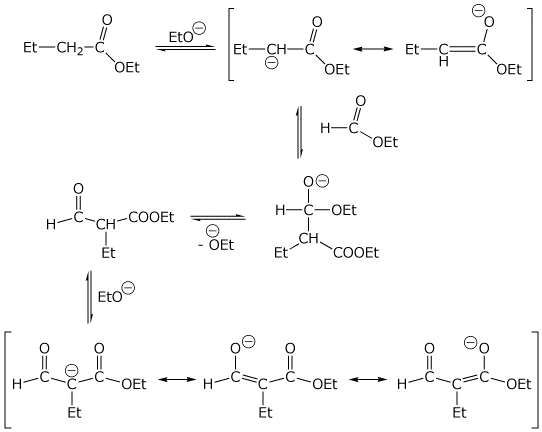
4. (4 points) Write a complete mechanism for the mixed Claisen condensation shown below. Write all steps and all resonance forms (including the resonance forms for the expected product before neutralization).