Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2007 |
Exam 3 Answer Key |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2007 |
Exam 3 Answer Key |
![]()
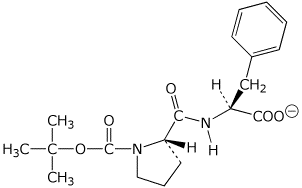
1. (20 points) Write accurate structures for the following, including clear stereochemistry:
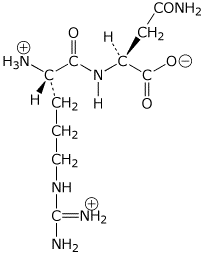
a) ArgAsn at pH 7
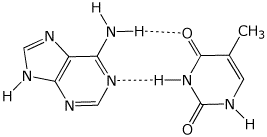
b) an H-bonded A - T base pair (just the bases)
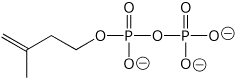
c) isopentenyl pyrophosphate ( at pH 7)
d) BOC-protected ProPhe (at pH 7)
2. (15 points) The pKa values for glutamic acid are 2.2, 4.3, and 9.7.
Write the expected major structures at
pH 1 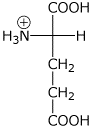
pH 3 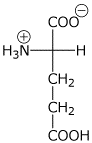
pH 7 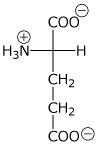
pH 10 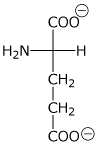
Explain why the two carboxy groups have different pKa values - including an explanation for why one is a stronger acid than the other.
3a. (10 points) Cardiolipin is common in heart muscle. The structure of a typical cardiolipin is shown below. Show the expected products from complete hydrolysis, including the number of moles of each product expected from one mole of cardiolipin.
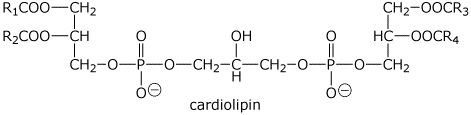
R1COOH, R2COOH, R3COOH, and R4COOH
HOCH2CHOHCH2OH
PO4-3
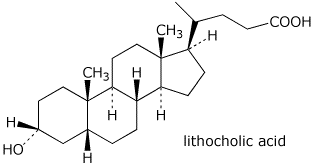
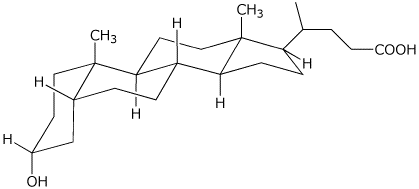
3b. (10 points) Lithocholic acid is a representative bile acid, used to solubilize fats. Place all of the substituents (10 of them) onto the chair diagram.
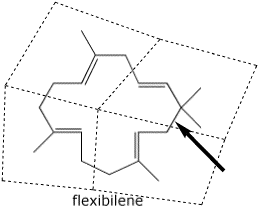
4. (10 points) Flexibilene, found in a marine coral, has an unusual 15-membered ring structure.
Circle each of the isoprene units in flexibilene.
Predict which bond is the last one made, creating the ring from an acyclic
precursor.
(place a clear arrow pointing to the bond)
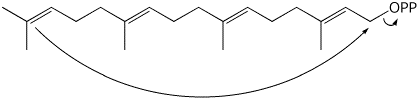
The acyclic precursor is a diterpene diphosphate. Predict its structure.
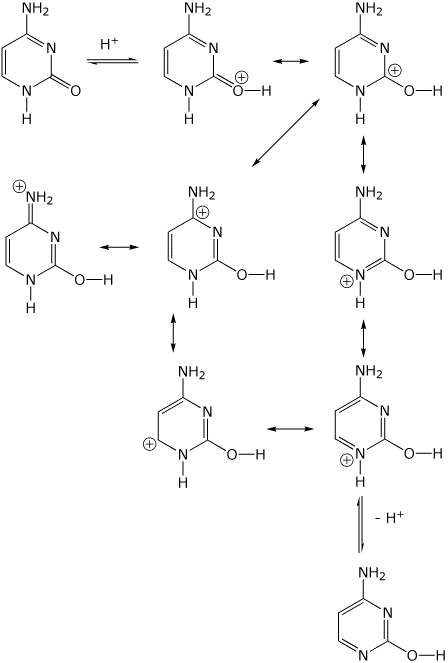
5. (20 points) The conversion of nucleic acids bases from their carbonyl to enol forms can be catalyzed by either acid or base. Start with cytosine (which has just one carbonyl) and show mechanisms for converting it to its enol form, using acid or base. Show all resonance forms for the intermediate in each case.
cytosine (carbonyl form) <======= (acid, H+ ) =======> cytosine (enol form)
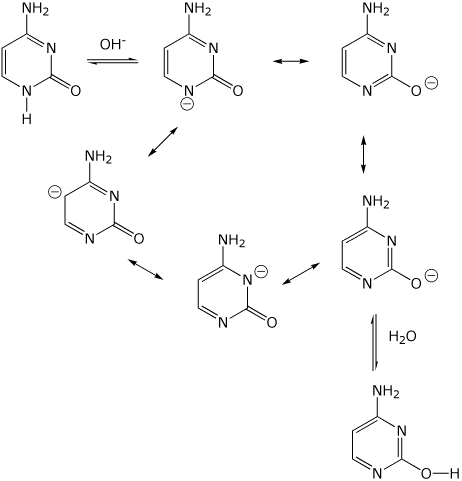
cytosine (carbonyl form) <======= (base, OH- ) =======> cytosine
(enol form)
6. (15 points) Identify the unknown heptapeptide based on the following data.
Complete hydrolysis gives one equivalent each of Arg, Asp, Gly, Pro, Ser, Tyr, Val
Edman degradation releases Asp
Carboxypeptidase releases Pro, then Gly
Chymotrypsin cleavage gives a tetrapeptide A and tripeptide B
Trypsin cleavage gives a tetrapeptide C and a tripeptide D
A and D have Asp at their N-termini
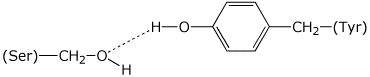
B has Val at its N-terminus and C has Tyr at its N-terminusAsp-Ser-Arg-Tyr-Val-Gly-Pro
Using only the amino acids found in the heptapeptide above, give examples of side chain interactions that illustrate
a) charged group attractions
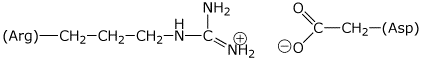
b) H-bonding attractions