Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2007 |
Exam 2 Answer Key |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2007 |
Exam 2 Answer Key |
![]()
1. (15 points) Write accurate names for the following:
a) 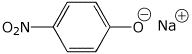
b) 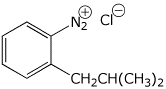
c) 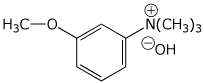
d) 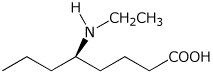
e) 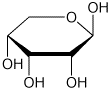
2. (15 points) Write accurate structures for the following:
a) N-methyl-N-nitrosoaniline
b) 2,5-dicyano-1,4-benzoquinone
c) N-benzyl-1-octadecanamine
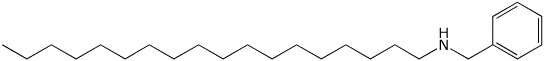
d) an L-ketopentose
e) beta-D-fructofuranose
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
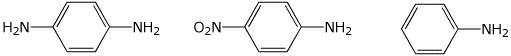
a) basicity
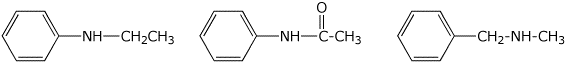
MIDDLE / / LEAST / / MOST
b) basicity
MOST / / LEAST / / MIDDLE
c) ease of hydrolysis in acid
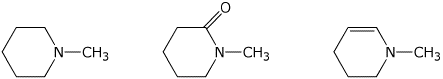
LEAST / / MIDDLE / / MOST
d) reactivity in nucleophilic substitution
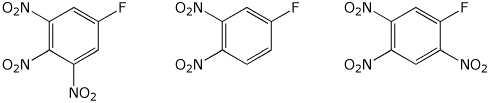
MIDDLE / / LEAST / / MOST
e) equivalents of HIO4 needed for complete reaction
![]()
MIDDLE / / MOST / / LEAST
4. (15 points) Write a complete mechanism for the acid-catalyzed hydrolysis of the compound shown below. Bring the sugar to its open-chain form. Show all steps and all resonance forms for any intermediates involved.

5. (10 points) Show a sequence of reactions that could be used to prepare p-bromobenzonitrile.
Show how the reaction sequence would differ if the target compound were m-bromobenzonitrile.
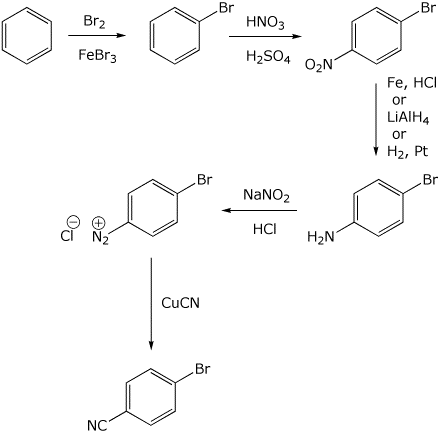
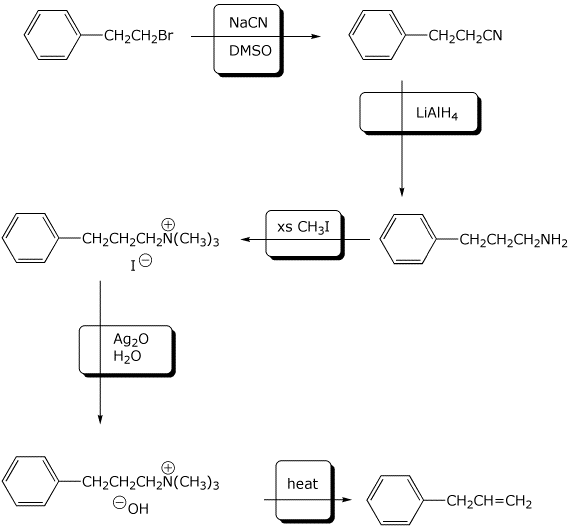
6. (10 points) Complete the following synthetic sequence by adding the missing reagents at each step.
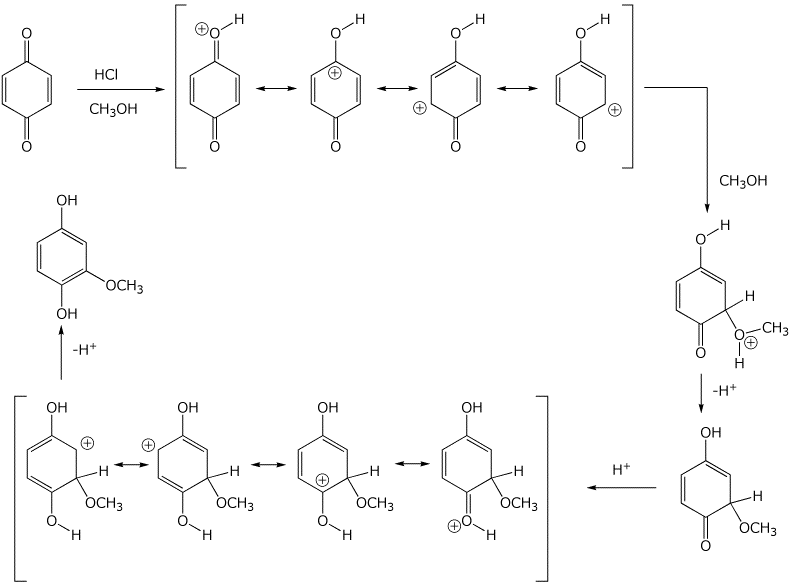
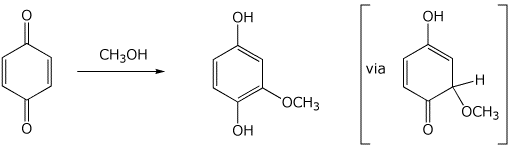
7. (20 points) A quinone may be converted to a hydroquinone by a nucleophilic addition reaction, as illustrated below. (Hint - think Michael addition)
Write two complete mechanisms - using base catalysis or using acid catalysis.
Show all steps and all resonance forms for any intermediates involved.
Base catalysis:
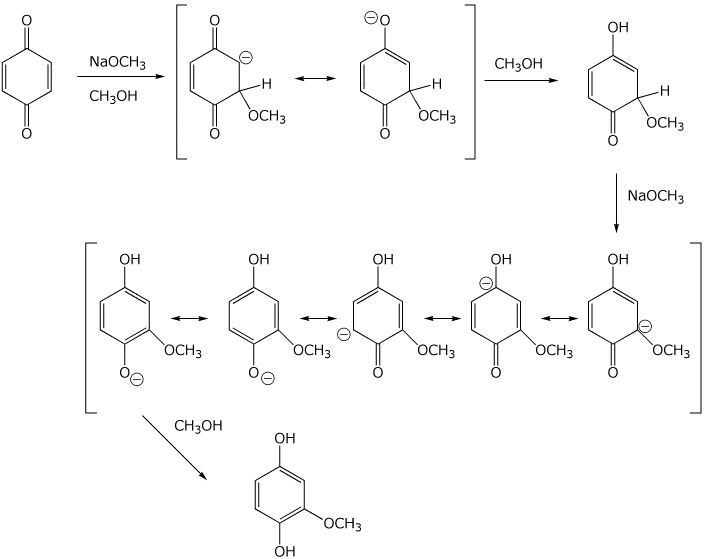
Acid catalysis: