Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2006 |
Quiz 21 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2006 |
Quiz 21 |
![]()
1. (3 points) Complete the following reactions:
a) 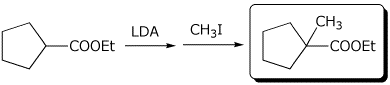
b) 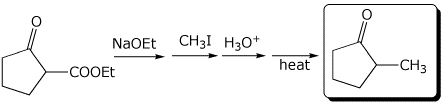
c) 
2. (3 points) Show all the steps (no mechanisms) that would be used in a malonic ester synthesis of hexanedioic acid, using 1,2-dibromoethane as the alkyl halide.

3a. (3 points) Write a complete mechanism (including all resonance forms for any intermediates involved) for the mixed Claisen condensation between diethyl oxalate and ethyl acetate (shown below).
![]()

3b. (1 point) Indicate the final product expected from the above Claisen condensation after hydrolysis and decarboxylation.
