Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2006 |
Exam 2 Answer Key |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring 2006 |
Exam 2 Answer Key |
![]()
1. (15 points) Give complete names for each of the following:
a) 
(S)-2-aminopropanal
b) 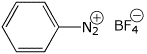
benzenediazonium fluoborate
c) 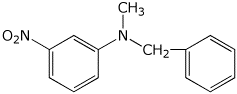
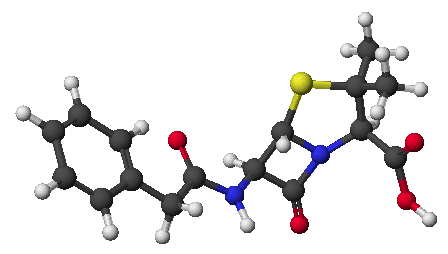
N-benzyl-N-methyl-3-nitroaniline
d) 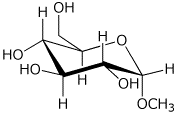
methyl alpha-D-glucopyranoside
e) 
alpha-D-ribofuranose
2. (15 points) Give complete structures for each of the following:
a) the enediol from glyceraldehyde
b) the C-4 epimer of D-glucose in beta-pyranose form
c) alpha-maltose (two D-glucose units joined alpha-1 to 4)
d) the benzyne from p-chlorophenol
e) the Hoffmann elimination product from tetrabutylammonium hydroxide
3. (15 points) Indicate the relative order of the following series of compounds with respect to the property listed. Write “MOST” and “LEAST” under the compounds with the highest and lowest values, respectively.
a) basicity
MIDDLE / / LEAST / / MOST
b) acidity
MOST / / LEAST / / MIDDLE
c) acidity
MOST / / MIDDLE / / LEAST
d) reactivity towards a nucleophile
LEAST / / MIDDLE / / MOST
e) ease of reduction
MIDDLE / / LEAST / / MOST
4. (15 points) Complete each of the following reactions by adding the major parts.
a) 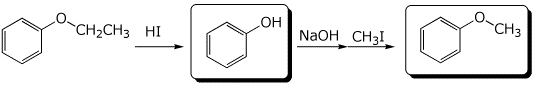
b) 
c) 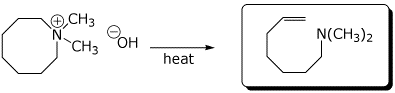
d) 
e) 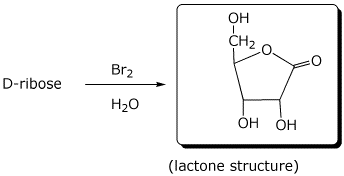
5a. (10 points) Show a synthetic sequence that could be used to prepare m-bromoacetanilide, starting from benzene and acetic acid.

5b. (10 points) In photosynthesis, shown below is the key step in which CO2 is taken in. Number the 5 carbons in the pentose in the standard way, call CO2 carbon 6, and illustrate where the six carbons are located in the next two steps.
Are these balanced reactions?
The first one is balanced, the second involves addition of H2O.
If not, indicate whether oxidation or reduction is taking place.
No overall oxidation or reduction is involved in either step.
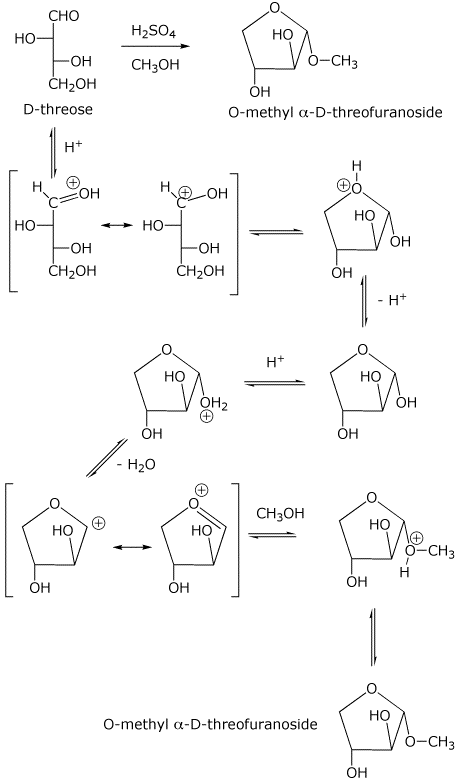
6. (20 points) The structure of D-threose is shown below.
a) (3 points) Write an accurate structure for the glycoside that would be formed in the reaction shown below.
b) (3 points) Give a complete IUPAC name for D-threose, including stereochemistry.
(2S,3R)-2,3,4-trihydroxybutanal
c) (4 points) Indicate the products that would be expected on treating D-threose with excess HIO4, and compare the products that would be obtained by treating the glycoside (above) with excess HIO4.
from D-threose --> 3 HCOOH + H2CO
from the glycoside -->
d) (10 points) Write a complete mechanism for the conversion of D-threose to the glycoside shown above using acid catalysis. Show all steps and all resonance forms for any intermediates involved.