
Lipids

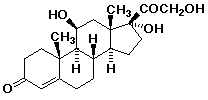
1. Draw a good conformational structure for cortisol, shown
below:
2. Dehydration of fats at high temperature (e.g., frying bacon) leads to acrolein
(propenal) as a volatile decomposition product. Propose a mechanism.
3. Write a complete IUPAC name for the monoglyceride that would be formed
between glycerol and oleic acid. Indicate stereochemistry and all of the various
possible isomers.
4. Glycerol is often used as a comonomer with ethylene glycol in a step reaction
polymerization with terephthalic acid. Show the repeat unit of the polymer
(without glycerol) and explain what differences in structure and properties
would be expected by inclusion of glycerol.
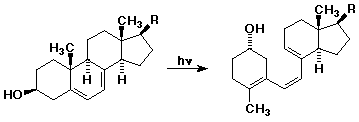
5. One of the key reactions in the biosynthesis of vitamin D (the sunshine
vitamin) involves the photochemical ring-opening of ergosterol as shown below.
Number the carbons and indicate the electron flow that leads to the reaction.
Describe the transition state and the observed stereochemistry.
6. Cobra venom contains a phospholipase that hydrolyzes the fatty acid ester
on C-2 of phospholipids. Write the structure of a typical lecithin before and
after this hydrolysis.
The effect of the hydrolysis is to destabilize the membrane structure. In general,
phospholipids (with two fatty acid "tails") form stable bilayers,
while simple detergents (with a single fatty acid "tail") form micelles.
Based on the structural differences, explain the different behaviors.
Materials adapted from:
Peer-Led Team Learning: Organic Chemistry, 1/e
Jack A. Kampmeier, University of Rochester
Pratibha Varma-Nelson, St. Xavier University
Donald Wedegaertner, University of the Pacific
Prentice-Hall, 2001, ISBN 0-13-028413-0
http://www.sci.ccny.cuny.edu/~chemwksp/OrganicChem.html



![]()

