
Amines, Aryl Halides, Phenols

1. Treatment of the compound shown below with iodomethane led to the formation
of two isomeric products, A and B. When each of these was treated
first with Ag2O/H2O followed by heating, there was formed the same mixture of
compounds C and D. Compound C could be resolved into enantiomers
but D could not.
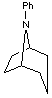
a. Provide structures for A and B and indicate the structural
relationship between them.
b. Provide structures for C and D.
2. Compound A, C7H15NO,
was neutral and exhibited strong absorption in the infrared spectrum at 1652
cm1 but none in the 3200-3400 cm1 region. Extended heating of A with
H3O+/H2O gave compound B and,
after addition of NaOH, a nitrogen-containing compound C. B exhibited
strong absorption in the infrared spectrum at 1712 cm1 and a very broad
absorption over the range 2500-3500 cm1. The 1-H NMR spectrum of B consisted
of the following absorptions: 1.20 ppm (d, rel. area 6, J = 7.0
Hz), 2.41 ppm (septet, rel. area 1, J = 7.0 Hz), and 12.40 ppm (s, rel.
area 1). Compound C exhibited a single absorption at 3335 cm1 in
the infrared spectrum and reacted with nitrous acid at 0 °C to give a yellow
oil. Compound C was synthesized by treating ethyl amine first with formic
acetic anhydride and then with LiAlH4 (H3O+
work up). Provide structures for A, B, and C which are
consistent with this evidence.
3. Compounds A, B, and C all have a molecular formula
of C10H12O2 and their IR and NMR spectra show that they are all p-disubstituted
benzenes. All of these compounds are water insoluble. Compound A is
insoluble in both NaOH and NaHCO3. Compound B is
soluble in both NaOH and NaHCO3, and compound C is
soluble in NaOH but insoluble in NaHCO3. Give structures
for A, B, and C, consistent with this information.
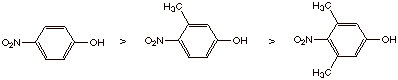
4. Explain the relative acidities of several phenols as shown below. (Hint:
Consider the structures of the phenolate ions.)
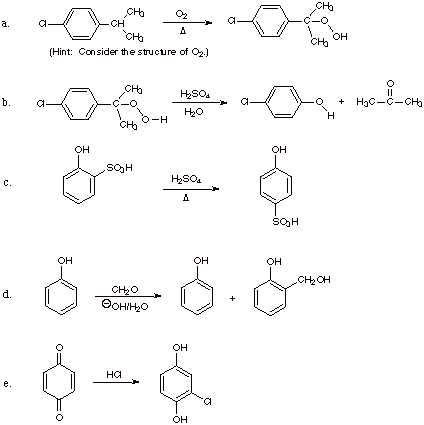
5. Give a reasonable mechanism for each the following reactions. Use curved
arrows to show movement of electron pairs and "fish hooks" to show
the movement of single electrons.
Materials adapted from:
Peer-Led Team Learning: Organic Chemistry, 1/e
Jack A. Kampmeier, University of Rochester
Pratibha Varma-Nelson, St. Xavier University
Donald Wedegaertner, University of the Pacific
Prentice-Hall, 2001, ISBN 0-13-028413-0
http://www.sci.ccny.cuny.edu/~chemwksp/OrganicChem.html


![]()


