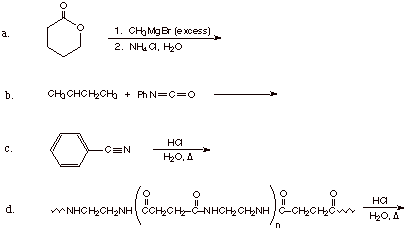Carboxyl derivatives

1. Provide structures that satisfy the following descriptions:
a. the structure of the compound, C8H10, that is converted upon vigorous oxidation with permanganate
to a compound, C8H6O4, which loses water upon heating.
b. the structure of the compound, C6H10O3, that exhibits strong absorption
in the infrared spectrum at 1754 cm-1 and 1818 cm-1 and the following
1-H NMR spectrum:
1.19 ppm (t, rel. area 3, J = 7.0 Hz) and 2.48 ppm (q, rel. area 2, J =
7.0 Hz).
c. the stereoisomer of 3-hydroxycyclohexanecarboxylic acid that is capable
of forming a lactone.
d. the structure of the compound, C6H12O2, that would undergo reaction with
excess ethyl magnesium bromide to give the compound shown below.
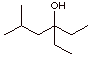
2. Propose a plausible mechanism for each of the following reactions. Provide
all important Lewis structures that contribute to the resonance hybrid for
delocalized intermediates, and point out any important driving forces.
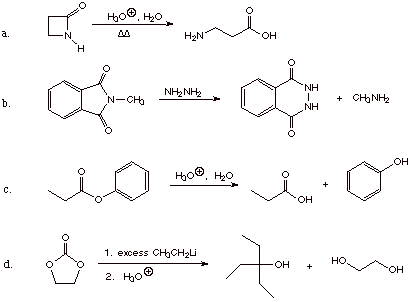
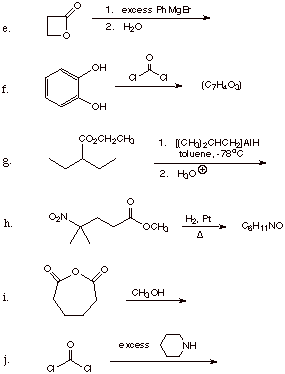
3. Give the structure(s) of the major organic product(s) for the following
reactions.
Materials adapted from:
Peer-Led Team Learning: Organic Chemistry, 1/e
Jack A. Kampmeier, University of Rochester
Pratibha Varma-Nelson, St. Xavier University
Donald Wedegaertner, University of the Pacific
Prentice-Hall, 2001, ISBN 0-13-028413-0
http://www.sci.ccny.cuny.edu/~chemwksp/OrganicChem.html



![]()