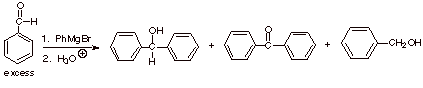Enols and Enolates

1. Show how to carry out the following chemical conversions using any
necessary organic and inorganic reagents. More than one synthetic step
may be required for a synthetic transformation.
2. Consider and explain the following observations.
If excess benzaldehyde is used in the reaction below, large amounts of
benzophenone and benzyl alcohol are formed, in addition to the expected
diphenylmethanol. Benzophenone and benzyl alcohol are formed in 1:1 yield.
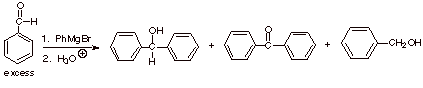
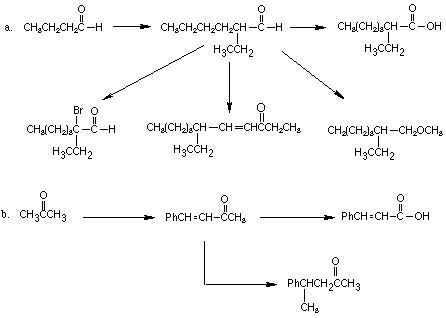
3. Give a reasonable mechanism for each of the following reactions, clearly
showing all important intermediates and resonance structures, and using
curved arrows to show movement of electron pairs.
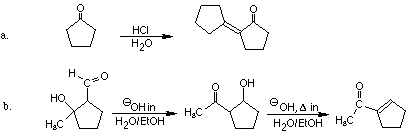
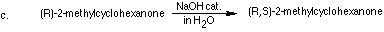
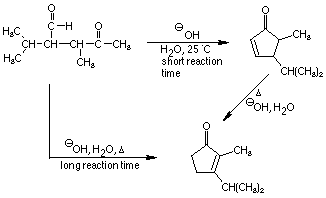
4. Give reasonable mechanistic pathways that account for the formation
of two products under different reaction conditions. Explain why each is
the preferred product under the reaction conditions specified.
Materials adapted from:
Peer-Led Team Learning: Organic Chemistry, 1/e
Jack A. Kampmeier, University of Rochester
Pratibha Varma-Nelson, St. Xavier University
Donald Wedegaertner, University of the Pacific
Prentice-Hall, 2001, ISBN 0-13-028413-0
http://www.sci.ccny.cuny.edu/~chemwksp/OrganicChem.html



![]()