|
Organic
Chemistry III
|
|
Professor Carl C. Wamser |
||
Quiz 26 |
Chem 336 - Spring 2005 |
1a. (2 points) Write an accurate structure for oleic acid (the C18 monounsaturated fatty acid).
1b. (2 points) If oleic acid were biosynthesized from labeled acetic acid, indicate which carbons of oleic acid would have the labels. (CH3-C*OOH, where C* is a C-14 label) Put asterisks (*) on your structure above.
1c. (2 points) Unsaturation is often detected quantitatively by bromine (or iodine) additions.Write the structure of the dibromo product expected from oleic acid, indicating the specific stereochemistry expected at each stereocenter.
2. (2 points) Identify the isoprene units within humulene (shown below) by circling each unit.
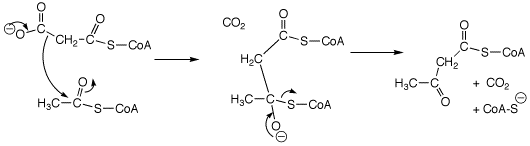
3. (2 points) Put appropriate electron-pushing arrows on the structures below to show the formation of the tetrahedral intermediate and the decomposition of the tetrahedral intermediate (also showing arrows) to give the indicated products.