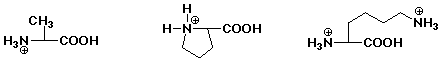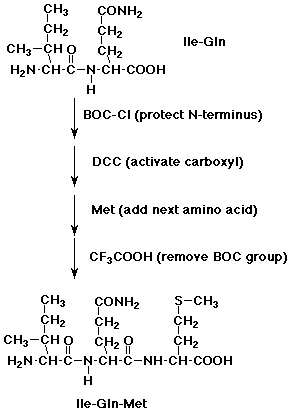Carey, pages 1152 - 1154 :
Problems 27.21 - 31
1. Write the structure of the tripeptide alanylprolyllysine, as it would
appear at pH 7.
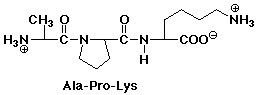
After acid hydrolysis into individual amino acids, show the products as
they would appear at pH 1.
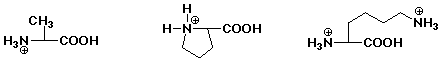
2. Show how you might start with a dipeptide, isoleucylglutamine, and specifically
add a methionine to the N-terminus.
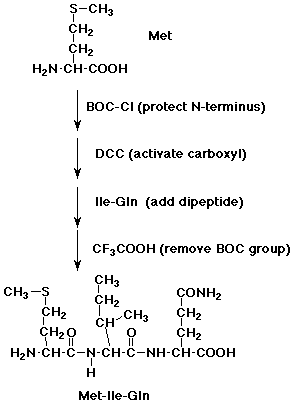
Repeat for the C-terminus.
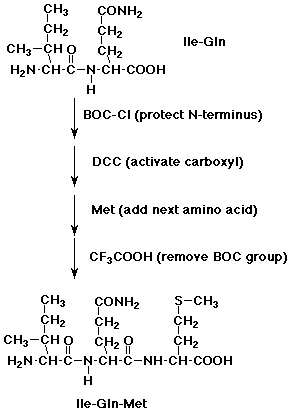
3. An unknown hexapeptide is determined by complete hydrolysis to have
the amino acid composition Ala (2), Lys, Glu, Phe, Cys.
Edman degradation releases Ala followed by Cys.
Chymotrypsin hydrolysis gives two tripeptides, both of which have Ala at
their N-termini.
Trypsin hydrolysis gives a pentapeptide plus Glu.
Identify the amino acid sequence of the hexapeptide.
Ala-Cys-Phe-Ala-Lys-Glu