
 |
Organic
Chemistry III
|
|
Professor Carl C. Wamser |
||
Exam 3 Answers |
Chem 336 - Spring 2005 |
1. (15 points) Write complete structures for each of the following, including all stereochemistry.
a) the methyl ester of methionine at pH 7
b) b-D-fructofuranose
c) the C-2 monoglyceride from hexadecanoic acid
d) TrpGlu at pH 7
e) the best chair form of methyl b-D-glucopyranose
2a. (9 points) Write all the resonance forms that illustrate the following:
a) why arginine is such a strong base
b) why the amide (peptide) bond has a barrier to rotation about the C-N bond
c) side-chain protonated histidine
2b. (6 points) NaBH4 reduction of D-fructose gives a mix of two epimeric
alditols.
Show their structures and the structures of the two aldoses that
would give these same alditols.
3a. (7 points) Show the location of all of the indicated substituents (including the H’s shown) on the chair structure of androsterone.
3b. (8 points) Show the products expected upon complete hydrolysis of the following:
4. (15 points) Complete each of the following reactions by adding the complete structure of the expected product, including all stereochemistry.
a)
Boc-Leu + DCC ----> 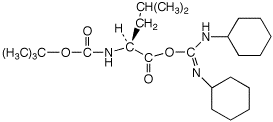
b) D-ribose + excess HIO4 ----> 4 HCOOH + CH2=O
c) L-glyceraldehyde + Cu+2
(Benedict’s reagent) ----> 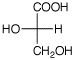
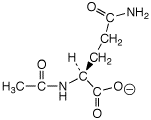
d) glutamine + acetic anhydride
----> 
e) alanine + Sanger’s reagent ----> 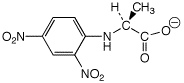
5. (15 points) Starting from the structure of D-talose shown below, write a pyranose form in both a and b anomers (using Haworth projections). For the b anomer, write both chair forms and predict the more stable one.
6. (10 points) For lysine, the pKa values are 2.18 and 8.95 for the a functional groups and 10.53 for the side chain. On the pH diagram below, indicate the structure expected to predominate in each of the lettered regions. Stereochemistry at the a position is not required.
7. (15 points) Use electron pushing arrows to show how NADH can be
used to reduce a ketone. Show the structures of the products expected.
What else that’s not shown would be useful to help catalyze this
reaction?
(besides enzyme magic)