|
Organic
Chemistry III
|
|
Professor Carl C. Wamser |
||
Exam 2 Answer Key |
Chem 336 - Spring 2005 |
1. (15 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 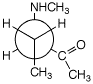
(R)-3-(N-methylamino)-2-pentanone
b) 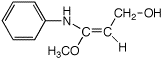
(E)-3-methoxy-3-(N-phenylamino)-2-propen-1-ol
c) 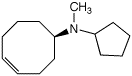
(R)-N-cyclopentyl-N-methyl-4-cyclooctenamine
2. (15 points) Write accurate structures for each of the following:
a) p-cyanobenzenediazonium fluoborate
b) N,N,N-trimethylanilinium chloride
c) N-methyl-N-nitroso-1-pentanamine
d) 2-amino-1,4-benzoquinone
e) N-propylphthalimide
3. (15 points) Complete each of the following reactions by adding the missing part: either the necessary reagents and conditions or the expected major product(s). If there are multiple organic products in the major reaction, show them all.
a) 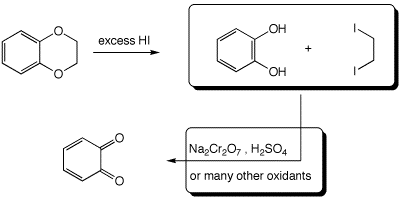
b)
c) 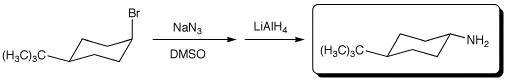
d) 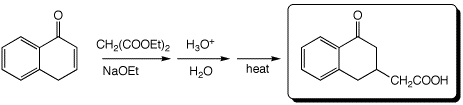
e) 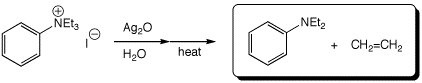
4. (15 points) Write a complete mechanism for the mixed Claisen condensation shown below. Show all steps and show all resonance forms. Show the compound that is formed prior to any exposure to acid.
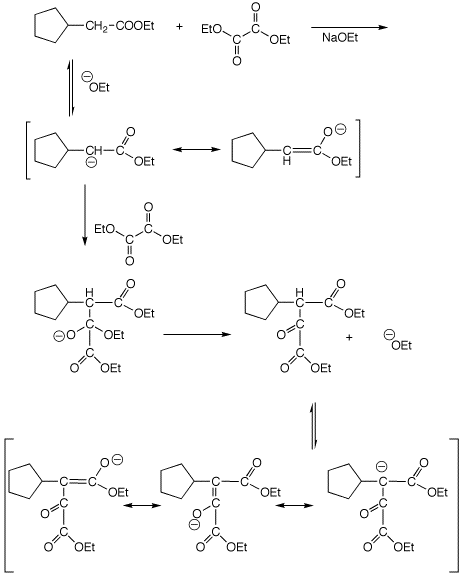
Show the final product that would be formed after acid hydrolysis and
decarboxylation.
(no mechanism needed)
![]()
5. (10 points) The aromatic nucleophilic substitution reaction shown below could proceed by either of two possible mechanisms. Write out both mechanisms and explain how you could tell which one actually occurred. Write only ONE resonance form for any intermediates.
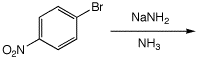
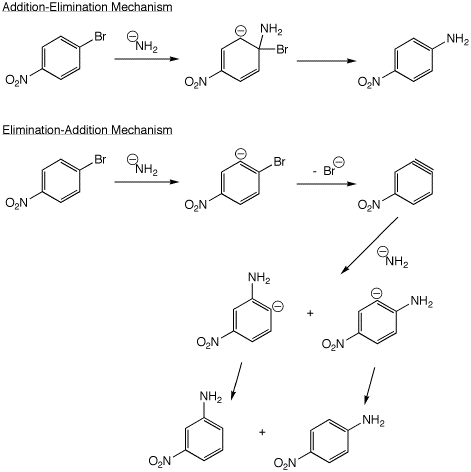
The elimination-addition mechanism will likely show both meta and para products, while the addition-elimination mechanism will show only the para product.
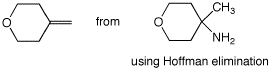
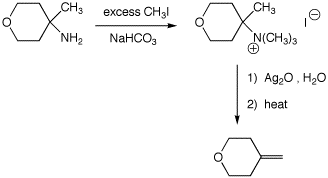
6. (30 points) Show appropriate sequences of reactions that could be used to prepare the indicated target compounds, starting with the indicated compounds as the sources of carbon.
a) 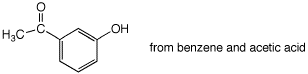
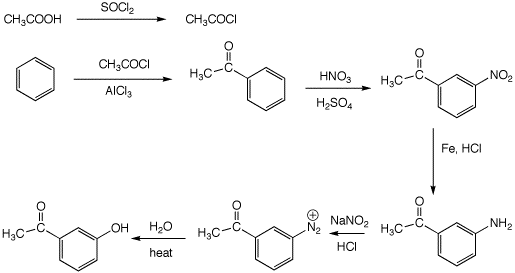
b) 
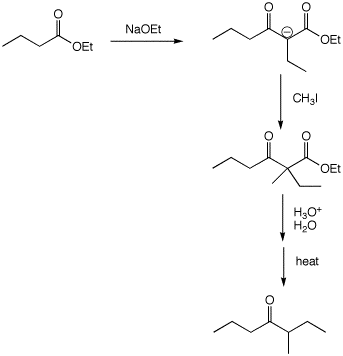
c)