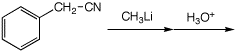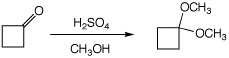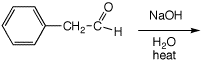1. (15 points) Write complete names for each of the following,
including stereochemistry if it is specifically shown.
a) 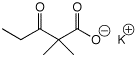
b) 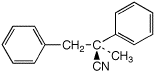
c) 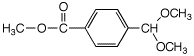
d) 
e) 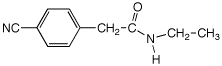
2. (15 points) Write accurate structures for each of the following:
a)
the hydrate of p-nitrobenzaldehyde
b) the enolate anion of tert-butyl
ethyl ketone
c) any delta-lactam
d) pentanedioic anhydride
e) cis-4-isobutylcyclohexanecarboxylic acid
3. (15 points) Write all the resonance forms for the intermediate that
would be formed in the first step of each of the following reactions.
(Just the step described - don’t try to finish the reaction.)
Add
H+ to the most basic site:
a) 
b) 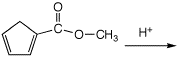
Remove H+ from the most acidic site:
c) 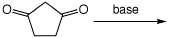
Add OH- to the beta-position:
d) 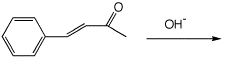
4. (15 points) Complete each of the following reactions by adding the
missing part: either the necessary reagents and conditions or the expected
major product.
a) 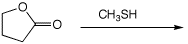
b) 
c) 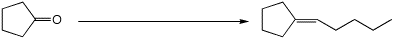
d) 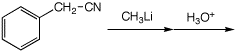
e) 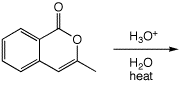
5. (15 points) Write a complete mechanism for the formation of the dimethyl
acetal of cyclobutanone. Show all steps and show all resonance forms for
any intermediates.
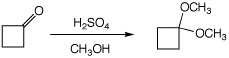
6. (10 points) Write a complete mechanism for the base-catalyzed aldol
condensation (with dehydration) of phenylacetaldehyde. Show all steps and
show all resonance forms for any intermediates.
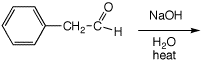
7. (15 points) The following retrosynthetic analysis was developed for
the preparation of the target compound phenylalanine. Show the sequence of
reactions from benzaldehyde that would be used to make phenylalanine.
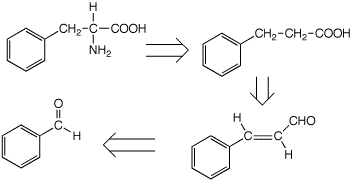






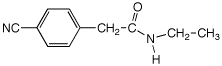
![]()
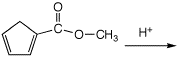
![]()

![]()
![]()
![]()