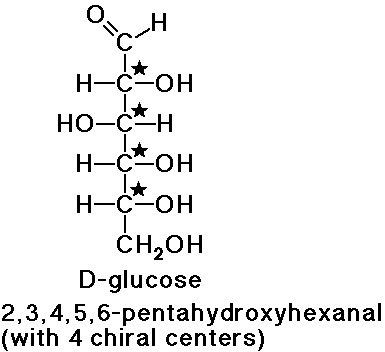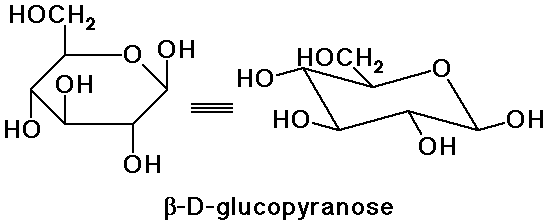Carbohydrates

Simple Sugars (Monosaccharides)
- general formula: (CH2O)n
- general structure: one carbonyl group, every other carbon has an
OH group
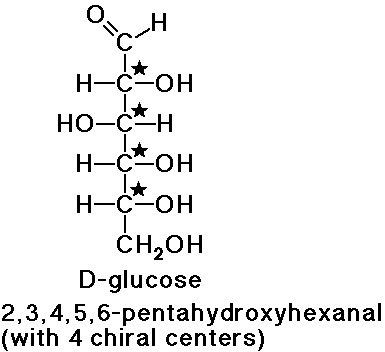
- monosaccharide classifications:
aldose: aldehyde
ketose: ketone
triose: 3 carbons
tetrose: 4 carbons
pentose: 5 carbons
hexose: 6 carbons
ketopentose: ketose with 5 carbons
etc.
Stereocenters
- D-glucose has 4 chiral carbon atoms (24 = 16 possible
stereoisomers)
the name D-glucose implies just one of those stereoisomers
one stereoisomer is the enantiomer of D-glucose
the other 14 stereoisomers are diastereomers of D-glucose
D-Glyceraldehyde
- the simplest chiral sugar
- the reference for D & L designation of stereochemistry
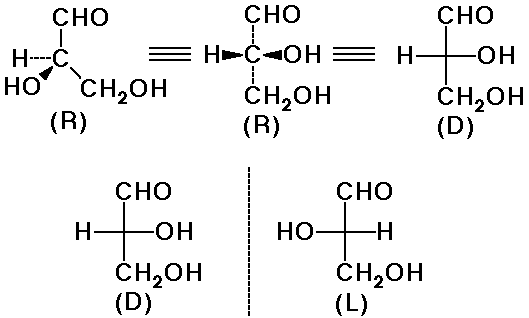
Writing D-Sugars
- orient the carbonyl end up
- write all chiral centers in Fischer projections
- D means the lowest OH group is right
- L means the lowest OH group is left
The D-Aldose Family
- trioses: D&L glyceraldehyde
- tetroses: D&L erythrose and D&L threose
- pentoses: 4 pairs of stereoisomers (including D-ribose)
- hexoses: 8 pairs of stereoisomers (including D-glucose)
Cyclic Sugar Structures
- internal hemiacetal formation
an alcohol group adds to the carbonyl
furanose: a 5-membered ring
pyranose: a 6-membered ring
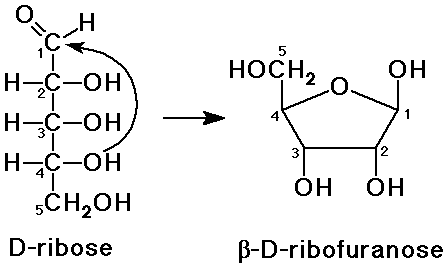
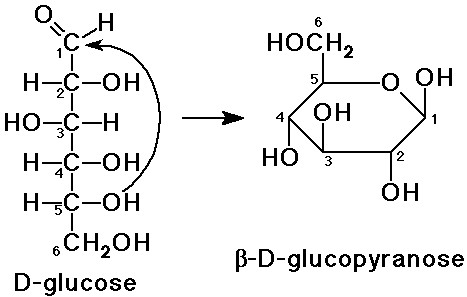
Haworth Projections
- a method to depict ring structures (flat)
arrange the ring with O in the back (or back-right)
- an OH to the right (Fischer) is down (Haworth)
- an OH to the left (Fischer) is up (Haworth)
- D-sugars will have the last CH2OH group up
- the new hemiacetal could have either configuration
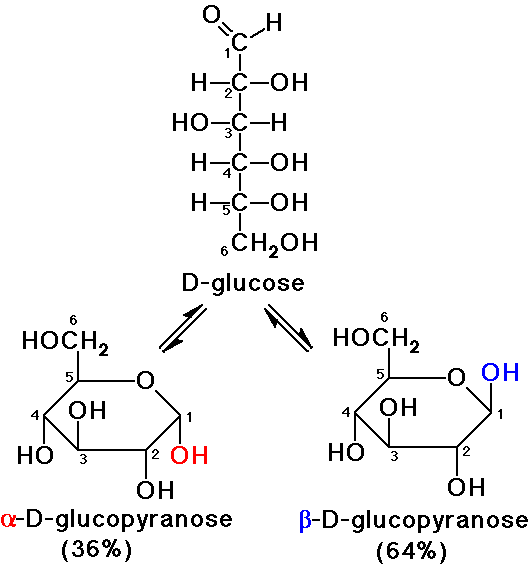
Anomers
- the two stereoisomers at the hemiacetal (anomeric) carbon
- alpha anomer: OH group is down (Haworth)
- beta anomer: OH group is up (Haworth)
- anomers are diastereomers (different physical properties)
Mutarotation of Glucose
- alpha-D-glucopyranose and beta-D-glucopyranose interconvert
starting with either one, a mixture results in solution
- actual structure of glucose in solution is about 64% beta, 36% alpha, <1%
open-chain
Conformations of Sugars
- 5-membered rings are close to planar
(Haworth projections are OK)
- 6-membered rings are chair conformations
(Haworth projections are inaccurate)
- from a Haworth 6-membered ring,
flex the right down and the left up
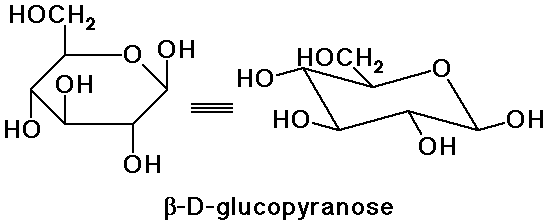
- beta-D-glucose has every substituent equatorial (most stable)
Reactions of Monosaccharides
- hemiacetals to acetals with alcohol + acid
(an acetal, rather than a hemiacetal, is called a glycoside)
- OH groups to esters with acetic anhydride
- OH groups to ethers with methyl sulfate (+ base such as NaH )
- reduction of the carbonyl with NaBH4 (polyalcohol is
called an alditol)
- oxidation of aldehydes to carboxylic acids with Tollen's reagent
(polyhydroxy carboxylic acid is called an aldonic acid)
- oxidation of aldehydes and primary alcohol (both ends) to acids
with HNO3 (polyhydroxy dicarboxylic acid is called an aldaric
acid)
Glycosides
- acetals do not interconvert with the open-chain form
(they don't mutarotate like hemiacetals)
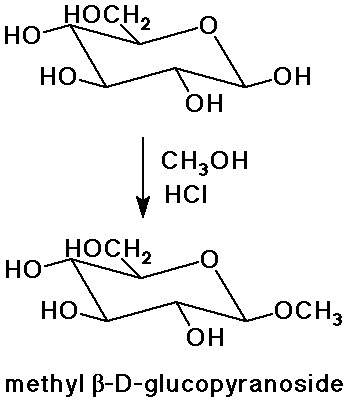
- glycoside linkages are used to connect sugars to other biomolecules,
including other sugars or nucleic acid bases
Reducing Sugars
- sugars that react positively with Tollen's reagent (Ag+)
or Cu+2
indicates a free aldehyde (or hemiacetal)
aldoses are reducing sugars
glycosides are not reducing sugars
ketoses are not reducing sugars
Periodate Cleavage Reactions
- periodic acid ( HIO4 or H5IO6 ) cleaves the C-C bond between an
alcohol and an adjacent alcohol or carbonyl group
- excess periodate can cleave apart all carbons of a sugar
- 1° alcohols oxidize to formaldehyde after cleavage
2° alcohols oxidize to aldehydes after cleavage
aldehydes oxidize to formic acid after clevage
ketones oxidize to carboxylic acids after cleavage
carboxylic acids oxidize to CO2 after cleavage
- a useful technique for decomposing sugars and deducing structure
Disaccharides
- linkage of two monosaccharides,
usually by glycoside (acetal) formation
Glucose Disaccharides - Maltose and Cellobiose
- maltose: glycoside link from a 4'-OH to make an acetal at C-1 (alpha)
a 1,4'-alpha-glycoside
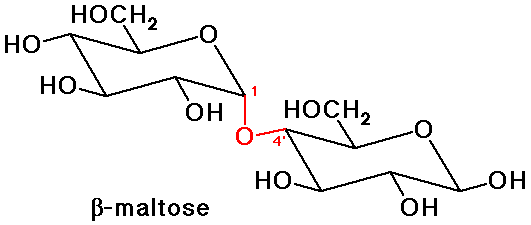
- cellobiose: glycoside link from a 4'-OH to make an acetal at C-1
(beta)
a 1,4'-beta-glycoside
Sucrose - A Disaccharide of Glucose + Fructose
- glucose C-1 and fructose C-2 join one another at their acetal carbons
sucrose is not a reducing sugar
Polysaccharides
- cellulose: long chains of glucose, joined 1,4'-beta
wood is mainly cellulose
- starches: storage form of glucose in plants
long chains of glucose, joined 1,4'-alpha (amylose)
some 1,6'-alpha linkages also occur (amylopectin)
we can digest alpha-linkages but not beta-linkages
- glycogen: storage form of glucose in animals
very long chains (1,4'-alpha), with lots of branching (1,6'-alpha)

![]()

![]()
![]()