
Aryl Halides & Phenols

Aryl Halides - Bonding
- resonance electron donation to aromatic ring makes C-X bond stronger
and less polar
- substitution reactions don't occur readily via SN1
or SN2 mechanisms
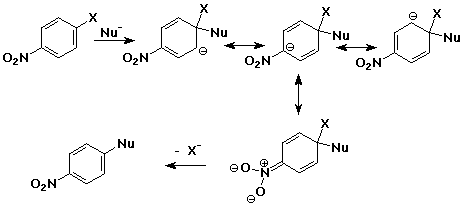
Aromatic Nucleophilic Substitution - addition/elimination mechanism
- addition of nucleophile to an aryl halide ( at the ipso position )
- intermediate is a delocalized anion, analogous to the cation in electrophilc
substitution
- usually works only with strong electron-withdrawing groups ortho & para
(e.g., nitro)
- loss of leaving group returns the aromaticity
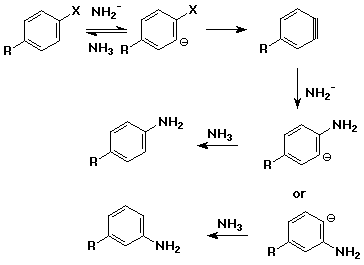
Aromatic Nucleophilic Substitution - elimination/addition (benzyne)
mechanism
- elimination from an aryl halide with a very strong base (usually NaNH2 in
NH3)
- intermediate is benzyne, highly reactive
- benzyne adds nucleophiles, e.g., NH3
- note different substitution patterns that can result
- benzyne also reacts in Diels-Alder reactions as a dienophile
Phenols - Bonding, Structure, and Properties
- resonance electron donation to aromatic ring makes C-O bond stronger
and less polar
- C-O bond is rarely broken
Acidity of Phenols
- O-H bond is more acidic than alcohols ( pKa ~ 10)
- phenols dissolve in NaOH solution (but not NaHCO3 -
distinguishing them from carboxylic acids)
- electron-withdrawing groups (especially o,p) increase acidity by stabilizing
phenolate anion
Reactions of Phenols
- reactive in electrophilic aromatic substitution (activating, o,p-directing)
- O-acylation (kinetic) vs. C-acylation (thermodynamic)
- Kolbe-Schmitt reaction: formation of salicyclic acid
- Claisen rearrangement: allyl aryl ether --> o-allylphenol
- aryl alkyl ethers can be cleaved by HI to phenols
- phenolate anion is a good nucleophile for Williamson ether synthesis
- oxidations of phenols generate quinones

![]()

![]()
![]()

