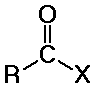Carboxylic Acids

Carboxylic Acids and Derivatives
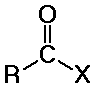
distinguished from aldehydes and ketones because one substituent is NOT C or
H
(usually Cl, O, or N, but it could also be other F, Br, I, S, P, or many other
possibilities)
Nomenclature of Carboxylic Acids
- alkanoic acid (-oic acid suffix)
when the -COOH is part of the parent chain
assumed #1 position
- cycloalkanecarboxylic acid
when the -COOH is not part of the parent name
assumed attached to the #1 position
- common names
formic acid (methanoic acid)
acetic acid (ethanoic acid)
notice the prefixes are the same as for aldehydes
- acyl group names
needed for naming some derivatives
formyl (methanoyl)
acetyl (ethanoyl)
- carboxylate salts
metal alkanoate (-oate suffix for anion)
Structure and Properties of Carboxylic Acids
- very polar functional group with H-bonding
typically the boiling points are relatively high
(formic acid, b.p. 100.7°)
Acidity of Carboxylic Acids
- stronger acids than alcohols
pKa ~ 5
(alcohol pKa values about 15-18 )
- electron-withdrawing effects of the carbonyl group tends to further
polarize the O-H bond so it is more easily ionized
- the carboxylate anion is stabilized by resonance
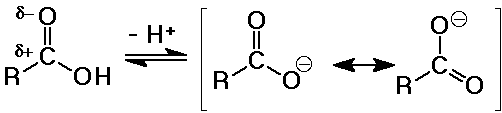
- substituent effects
electron-withdrawing groups (like Cl) increase acidity
Carboxylate Salts
- carboxylic acids generally dissolve in weak base (NaHCO3)
- salts are water-soluble for the smaller carboxylic acids
- salts of the large carboxylic acids are soaps
soaps have a hydrophilic (ionic) part
and a hydrophobic (hydrocarbon) part
Spectroscopy of Carboxylic Acids
- mass spec - alpha cleavage & McLafferty rearrangement
- NMR - COOH at 10-13 ppm (H) or 160-180 ppm (C-13)
- IR - broad C=O at about 1700 cm-1 and very broad O-H at about 3000-3400
cm-1 (H-bonding)
Synthesis of Carboxylic Acids
- oxidations
primary alcohols to carboxylic acids using CrO3 ,
other oxidants
aldehydes to carboxylic acids using Tollen's reagent (Ag+)
- hydrolysis of nitriles
alkyl halide to nitrile by SN2 substitution
followed by hydrolysis with acid or base catalysis
- addition of CO2 to a Grignard reagent
Reactions of Carboxylic Acids
- reduction to primary alcohols by LiAlH4
unreactive with catalytic hydrogenation or with NaBH4
- conversion to acid chlorides with SOCl2
conversion to other derivatives via the acid chloride
- diazomethane reaction - methyl esters
Fischer esterification
- conversion to esters with alcohol and acid catalysis
- a reversible equilibrium
favored to the right with excess alcohol
favored to the left with excess water (hydrolysis)
- see the complete mechanism in the homework answers
Decarboxylation
- beta-keto acids and beta-dicarboxylic acids lose CO2 readily
cyclic H-bonded transition state requires a beta C=O

![]()

![]()
![]()