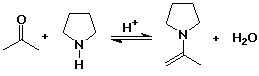Enols and Enolates

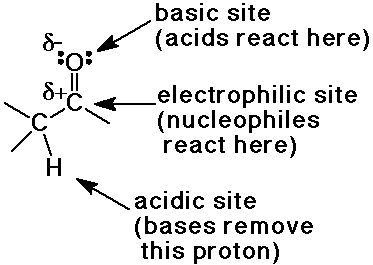
Reactive Sites of the Carbonyl Group
Enols
- tautomers - constitutional isomers that are easily interconverted
enol structure vs. carbonyl (keto) structure differs by location of one H
(and double bond)
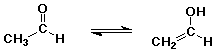
- most carbonyl and carboxyl compounds are in equilibrium with just small
amounts of enol
- enol form is favored only in special cases if the C=C double bond or
the O-H group is specially stabilized
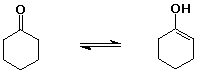
cyclohexanone (1 enol per million ketone molecules)
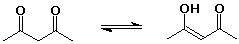
24% 2,4-pentanedione : 76% 4-hydroxy-3-penten-2-one
- knowing the right keto-enol forms for the nucleic acid bases allowed
Watson and Crick to develop the double-helix concept for DNA
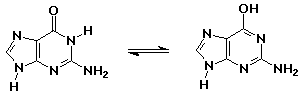
guanine (keto form - as in DNA) // guanine (enol form - aromatic but less
stable)
Acid- and Base-Catalyzed Enolization
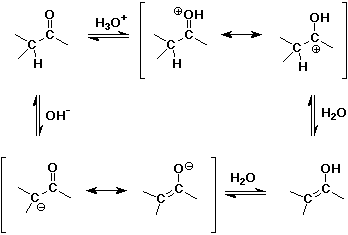
Alpha-Substitution on Enols
- enols behave like C=C double bonds - react with electrophiles
the net reaction is alpha-substitution
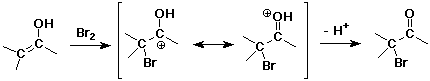
Enolate Anions
- carbonyl compounds have pKa values about 16 - 20
- strong bases (that are not good nucleophiles) can deprotonate carbonyl
compounds completely (LiN(iPr)2)
- NaOH deprotonates partially, but sufficient to initiate some reactions
(aldol)
- two carbonyl groups increases the acidity further
pKa of 1,3-diketones is about 9
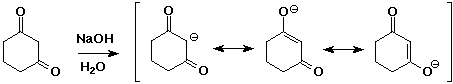
- enolates are good nucleophiles
Alpha-Halogenation
- halogens are readily substituted by acid or base catalysis
with acid - single substitution on the more highly substituted alpha carbon
with base - multiple substitutions on the less substituted alpha carbon
- haloform reaction - methyl ketones can be cleaved to haloform ( CHX3 )
Other Alpha-Substitution Reactions
- chiral alpha-carbons can be racemized by acid or base
- deuterium can be exchanged for hydrogen using D2O
The Aldol Condensation
- reaction of an enolate as nucleophile with a carbonyl group,
usually from the same compound
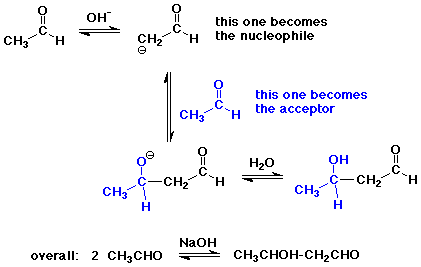
- the reaction is a reversible equilibrium
favored towards product (aldol) only for simple aldehydes
greater substitution favors starting compounds at equilibrium
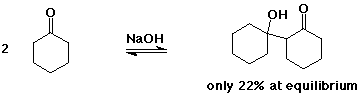
Dehydration
- the aldol reaction is often combined with dehydration of the initial
aldol product, forming a conjugated double bond (an enone)
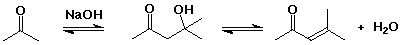
- base-catalyzed elimination via enolate anion (loss of OH-)
- acid-catalyzed elimination via enol (allylic cation intermediate)
Other Aldol Combinations
- mixed aldol reaction - two different carbonyl compounds
works best of one has no alpha-hydrogens, e.g., formaldehyde or benzaldehyde
- intramolecular aldol - dicarbonyl compound gives cyclic product
Enamines
- N analog of enols
- formed from 2° amines plus carbonyl compounds
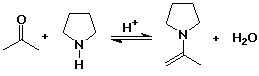
- the N lone pair makes the alpha position electron rich (nucleophilic)
- enamines react with alkyl halides (alkylation) or acyl halides (acylation)
- after hydrolysis, the carbonyl group is reformed
Alpha,Beta-Unsaturated Carbonyls
- electron-withdrawing effects of the carbonyl group make the C=C bond
reactive towards nucleophiles
- addition of a nucleophile to the beta-position of a conjugated a,b-unsaturated
carbonyl compound
creates another enolate anion (conjugate addition)
Michael Addition
- conjugate addition of a carbon nucleophile
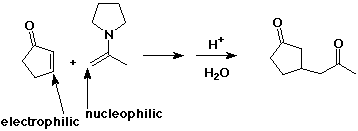
- organocopper reagents also do conjugate addition
- organolithium and Grignard reagents usually do direct carbonyl addition
( 1,2 addition )
these are rapid, irreversible additions under kinetic control
- conjugate addition products (addition to C=C rather than to C=O) are
more stable
- Robinson annulation - intramolecular Michael addition to form a ring
Other Related Condensation Reactions
- alpha-anions (nucleophiles) can be made next to any good electron-withdrawing
group
e.g., nitromethane
- these anions can react with carbonyl compounds to give addition
Biological Aldol Reactions
- aldolase is an enzyme that adds dihydroxyacetone to glyceraldehyde
to form fructose
two 3-carbon sugars join to become a 6-carbon sugar

![]()

![]()
![]()

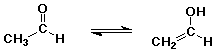
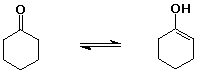
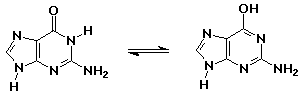

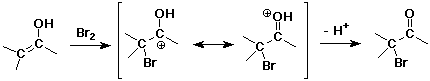
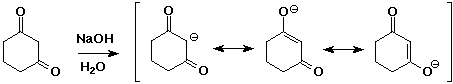

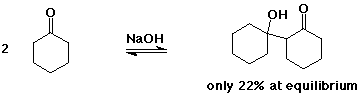
![]()