
Organic Chemistry III
Professor Carl C. Wamser

Chapter 27 Workshop Problems
Organic Chemistry III |
||
Professor Carl C. Wamser |
![]()
Organic Chemistry III |
||
Professor Carl C. Wamser |
![]()
![]()
1. Identify the expected state of ionization of the following in aqueous solutions of pH 4, 7, and 10.
a) amino acids - threonine, asparagine, tyrosine, histidine, and proline
b) peptides - isoleucylarginine, AlaTrpGluLeu, polyglycine
2. The name for the amino acid threonine comes from its similarity in structure to the sugar D-threose.
Write the structure for threonine in a Fischer projection that clearly shows
its similarity to D-threose.
Identify each stereocenter in threonine as R or S.
Only one other amino acid (of the 20 common ones in your table) has two stereocenters.
Which one? Write its structure with both stereocenters in the S configuration.
3. Consider an unknown heptapeptide consisting of the following amino acids:
Arg, Asp, His, Ile, Phe, Tyr, Val
Deduce its primary structure from the following data:
Edman degradation releases Asp
carboxypeptidase releases Phe
chymotrypsin cleaves it to a tripeptide and a tetrapeptide, A and B
A has Ile at its N-terminus and Phe at its C-terminus
B can be partially hydrolyzed to give dipeptides Arg Val, AspArg and ValTyr
Use the amino acids in this peptide to illustrate the following aspects of
tertiary structure.
Draw just the specific side chain structures involved.
a) electrostatic attraction
b) H-bonding
c) hydrophobic interactions
4. Illustrate the steps in the synthesis of the tripeptide AlaGlnLeu.
Predict the isoelectric point for this tripeptide.
What would be the mRNA code for this sequence?
5. Recreate the chymotrypsin template on the board and trace the steps in peptide hydrolysis.
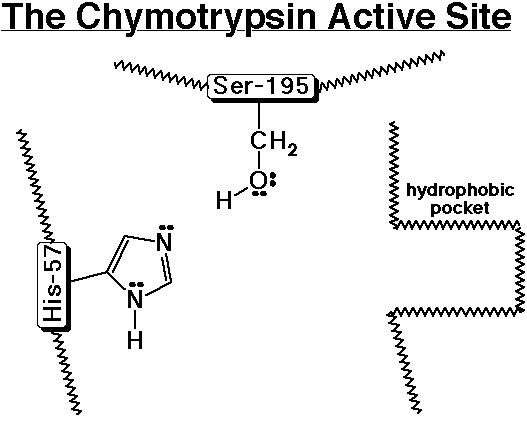
1) A polypeptide enters the active site and the aromatic amino acid side chain nestles into the hydrophobic pocket.
2) Ser-195 acts as a nucleophile to add to the carbonyl group of the peptide, while His-57 removes a proton from Ser-195 to make it a better nucleophile.
3) The tetrahedral intermediate splits out an amino group, while His-57 donates a proton to the amino group to make it a better leaving group.
4) The broken peptide fragment (the N-part) leaves the active site and is replaced by water.
5) Water acts as a nucleophile to add to the carbonyl group of the intermediate (which is now an ester), while His-57 removes a proton from water to make it a better nucleophile.
6) The tetrahedral intermediate splits out the Ser-195 group, while His-57 donates a proton to Ser-195 to make it a better leaving group.
7) The other part of the broken peptide (the C-part) leaves the active site and is replaced by another polypeptide (same as step 1 above).
Peer-Led Team Learning: Organic Chemistry, 1/e
Jack A. Kampmeier, University of Rochester
Pratibha Varma-Nelson, St. Xavier University
Donald Wedegaertner, University of the Pacific
Prentice-Hall, 2001, ISBN 0-13-028413-0
http://www.sci.ccny.cuny.edu/~chemwksp/OrganicChem.html