Organic Chemistry III
Professor Carl C. Wamser

Chapter 18 Quiz
Organic Chemistry III |
||
Professor Carl C. Wamser |
![]()
Organic Chemistry III |
||
Professor Carl C. Wamser |
![]()
![]()
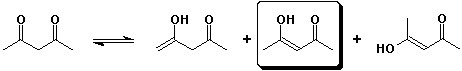
1. (3 points) Including stereoisomers, there are three possible enols of
2,4-pentanedione.
Write structures of all of them and circle the most stable enol.
2. (4 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Include stereochemistry if it is specific.
a) 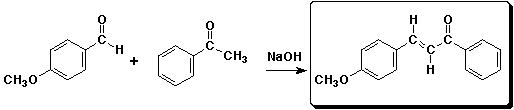
b) 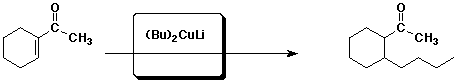
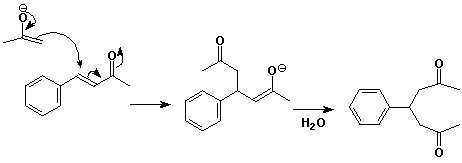
3. (3 points) The compound shown below is made from a Michael reaction of an enolate anion and an a,b-unsaturated ketone. Show the starting compounds that would be needed and use electron-pushing arrows to show how the key carbon-carbon bond-forming reaction proceeds.