 |
|
|
Organic Chemistry III
|
Professor Carl C. Wamser
|

Homework, Chapter 17

Carey, pages 745 - 754
Problems 17.20 - 26 , 28 - 43 , 45 - 49
1. Write complete names for each of the following:
a) 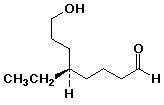
b) 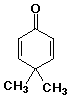
2. Write a complete mechanism for the acid-catalyzed formation of the cyclic
acetal between acetone and cis-1,2-cyclopentanediol.
Explain why an acetal is not formed with the trans isomer.
3. Develop a synthetic sequence for 3-ethyl-3-hexanol that uses as organic starting
materials only alcohols having four carbons
or fewer.
4. Explain how acetone in the presence of radioactively-labelled
water (H2O-18) slowly becomes radioactive
itself.



![]()
![]()

