
Organic Chemistry III
Professor Carl C. Wamser

Exam 2
Answer Key
Organic Chemistry III |
||
Professor Carl C. Wamser |
![]()
Organic Chemistry III |
||
Professor Carl C. Wamser |
![]()
![]()
1. (12 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 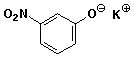
potassium 3-nitrophenolate
b) 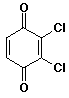
2,3-dichloro-1,4-benzoquinone
c) 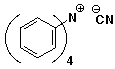
tetraphenylammonium cyanide
d) ![]()
N,N-dimethyl-4-nitrosoaniline
2. (15 points) Write accurate structures for each of the following.
a) malonic ester
b) LDA
c) p-phenylbenzenediazonium chloride
d) the transition state for decarboxylation of acetoacetic acid
e) the Diels-Alder product of benzyne and furan
3. (15 points) Within each
series below, place the compounds in order with respect to the property
indicated.
Write “MOST” and “LEAST” under the
compounds with the highest and lowest values of that property.
a) acidity
LEAST / / MOST / / MIDDLE
b) basicity
MIDDLE / / MOST / / LEAST
c) boiling point
MIDDLE / / LEAST / / MOST
d) rate of formation of benzyne
LEAST / / MIDDLE / / MOST
e) rate of nucleophilic substitution
MOST / / MIDDLE / / LEAST
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 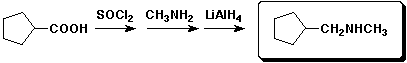
b) 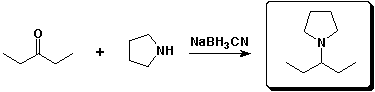
c) 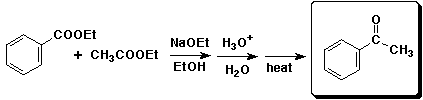
d) 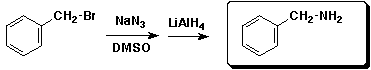
e) 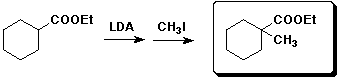
5. (10 points) Write all the resonance forms for the conjugate base that would be formed by deprotonation of each of the following.
a) 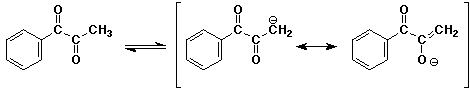
b) 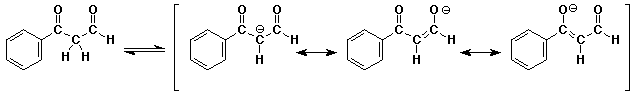
c) 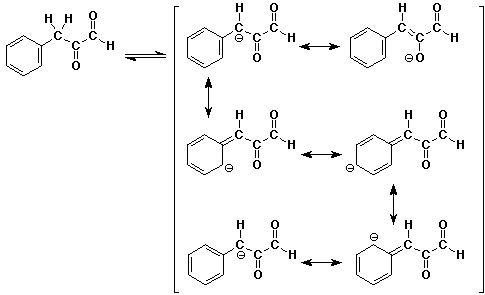
d) (3 points) Which of the above is the strongest acid?
b - two resonance forms on O
6. (10 points) Complete the following sequence of reactions by adding the missing parts:
7a. (10 points) Show the sequence of reactions that would be used in the synthesis of p-bromobenzonitrile starting from benzene.
7b. (10 points) Show the sequence of reactions that would be used in the synthesis of 2-methylbutanoic acid starting from malonic ester.