Organic Chemistry III
Professor Carl C. Wamser

Exam 1
Answer Key
Organic Chemistry III |
||
Professor Carl C. Wamser |
![]()
Organic Chemistry III |
||
Professor Carl C. Wamser |
![]()
![]()
1. (15 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 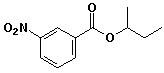
sec-butyl m-nitrobenzoate
or
1-methylpropyl 3-nitrobenzoate
b) 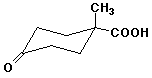
1-methyl-4-oxo-cyclohexanecarboxylic acid
c) ![]()
(R)-4-hydroxy-6-methylheptanenitrile
d) 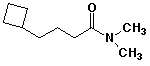
4-cyclobutyl-N,N-dimethylbutanamide
e) 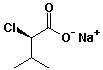
sodium (R)-2-chloro-3-methylbutanoate
2. (15 points) Write accurate structures for each of the following.
a) a beta-lactam
b) the dimethyl acetal of benzaldehyde
c) the Baeyer-Villager oxidation product from cyclopentanone
d) the neutral tetrahedral intermediate in the hydrolysis of methanamide
e) the most stable enol of 2-methylcyclohexanone
3. (15 points) Within each
series below, place the compounds in order with respect to the properety
indicated.
Write “MOST” and “LEAST” under the
compounds with the highest and lowest values of that property.
a) acidity
MIDDLE / / LEAST / / MOST
b) amount of enol at equilibrium
LEAST / / MIDDLE / / MOST
c) amount of hydrate at equilibrium
MOST / / MIDDLE / / LEAST
d) reactivity towards hydrolysis in acid
MIDDLE / / LEAST / / MOST
e) acidity
MOST / / LEAST / / MIDDLE
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 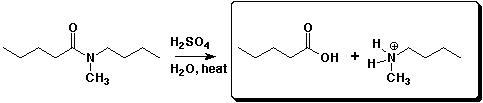
b) 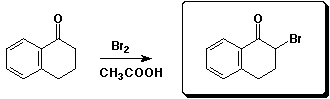
c) 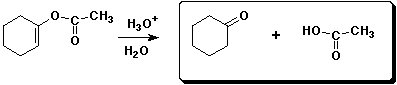
d) 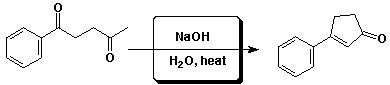
e) 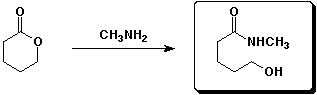
5. (15 points) Write a complete mechanism for the base-catalyzed aldol reaction
shown below.
Show all steps and all resonance forms for any intermediates involved.
6. (15 points) Write a complete mechanism for the acid-catalyzed reaction
shown below.
Show all steps and all resonance forms for any intermediates involved.
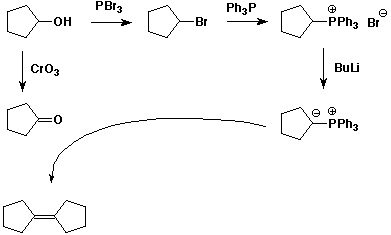
7. (10 points) Show the steps that would be used in the following synthesis.
Start from cyclopentanol only and include a Wittig reaction.