
Chem 336 - Spring 2002
Organic Chemistry III
Dr. Carl C. Wamser

Carey, pages 768 - 773:
Problems 19.13 - 19.34
1. Give IUPAC names for the following:
a) 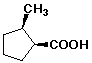
b) 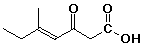
2. Write a complete mechanism for the acid-catalyzed hydrolysis
of methyl acetate.
The mechanism is the exact reverse of Fischer esterification.
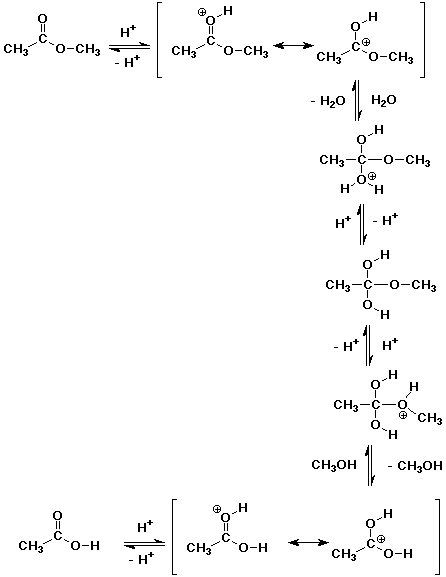
3. Show two different ways to prepare cyclohexanecarboxylic acid starting with cyclohexane.
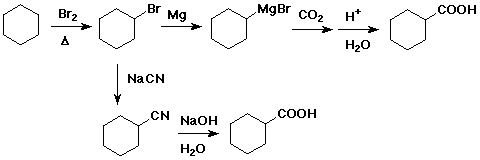
The upper pathway is likely to be better because the SN2 displacement by CN- on the 2° alkyl bromide may be complicated by some elimination.