
Chem 336 - Spring 2002
Organic Chemistry III
Dr. Carl C. Wamser

Carey, pages 691 - 700:
Problems 17.19 - 49
1. Write complete names for each of the following:
a) 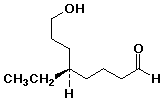
b) 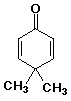
2. Write a complete mechanism for the acid-catalyzed formation
of the cyclic acetal between acetone and cis-1,2-cyclopentanediol.
Explain why an acetal is not formed with the trans isomer.
3. Develop a synthetic sequence for 3-ethyl-3-hexanol that uses
as organic starting materials only alcohols having four carbons
or fewer.
4. Explain how acetone in the presence of radioactively-labelled
water (H2O-18) slowly becomes radioactive
itself.