Chem 336 - Spring 2002
Organic Chemistry III
Dr. Carl C. Wamser

1. (15 points) Write complete names for each of the following:
a) 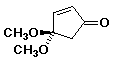
4,4-dimethoxy-2-cyclopentenone
b) ![]()
1-propylcyclobutanecarboxaldehyde
c) 
ethyl 6,6,6-trifluoro-5-oxohexanoate
d) 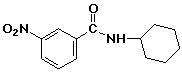
N-cyclohexyl-3-nitrobenzamide
e) 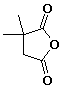
2,2-dimethylbutanedioic anhydride
2,2-dimethylsuccinic anhydride
2. (15 points) Arrange the following sets of compounds in order with respect to the property indicated. Write "MOST" and "LEAST" under the compounds with the highest and lowest values of the property.
a) acidity
CF3COOH / / CH3COOH / / CH3COOEt
MOST / / MIDDLE / / LEAST
b) acidity
MIDDLE / / MOST / / LEAST
c) reactivity towards hydrolysis
LEAST / / MOST / / MIDDLE
d) reactivity towards nucleophilic attack
LEAST / / MIDDLE / / MOST
e) reactivity towards nucleophilic attack
MIDDLE / / LEAST / / MOST
3. (15 points) Complete each reaction by adding the missing part: either the starting materials, the necessary reagents and conditions, or the final major product. Include stereochemistry if it is specific.
a) 
b) 
c) 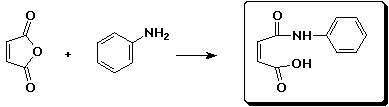
d) 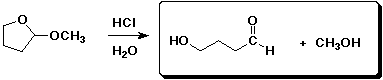
e) 
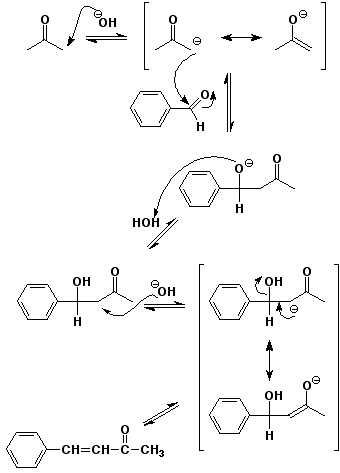
4. (15 points) Write a complete mechanism for the Claisen-Schmidt
condensation (mixed aldol reaction, including dehydration) of
benzaldehyde with acetone in aqueous base.
Show all steps and all resonance forms for any intermediates involved, and show electron-pushing arrows in each step.
5. (10 points) Write a synthetic sequence of reactions that could be used to make the compound shown below using ethanol as the only source of carbon.
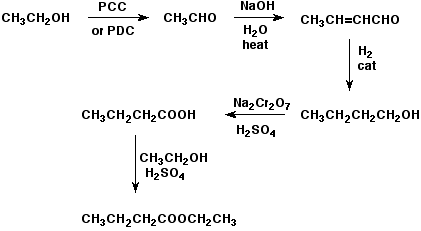
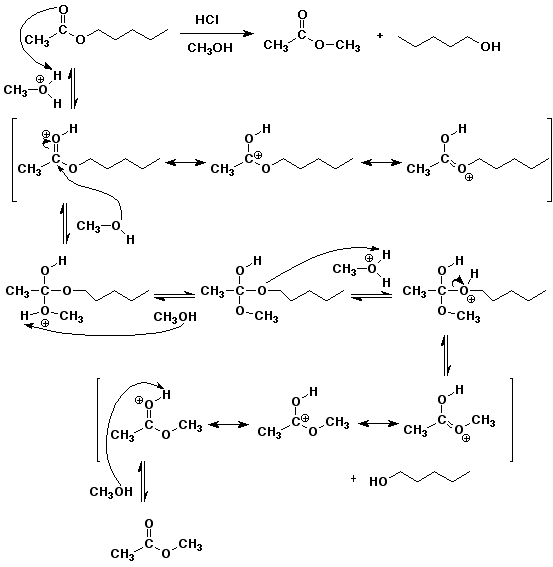
6. (20 points) Write a complete mechanism for the acid-catalyzed transesterification that converts pentyl acetate to methyl acetate by heating in acidic (HCl) methanol solution.
Show all steps and all resonance forms for any intermediates involved, and show electron-pushing arrows in each step.
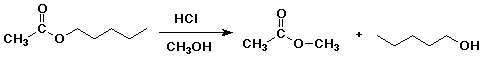
7. (10 points) Write the structures of the two carbonyl compounds that would be in equilibrium with the enol shown below. One is significantly more stable - predict which one and explain why.
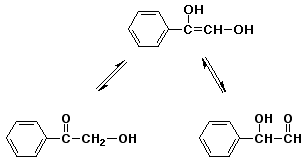
More stable
carbonyl is
conjugated with
the aromatic ring