
Chapter 27 Notes - Proteins and Nucleic Acids

Amino Acids
- natural amino acids are alpha (position of the amine) and L (stereochemistry)
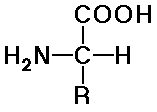
- structures differ by the R groups
there are 20 common natural amino acids
10 amino acids are essential to human nutrition
(we can biosynthesize the others)
Side Chains
- include a great variety of functional groups and properties
- glycine has no side chain
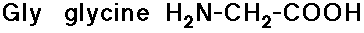
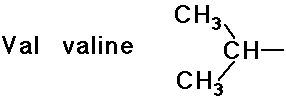
- simple alkyl groups (nonpolar)
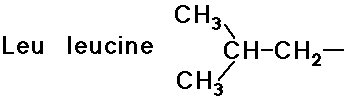
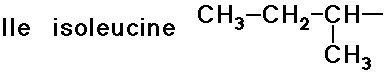
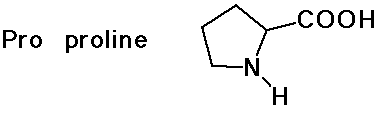
- cyclic side chain (a secondary amine)

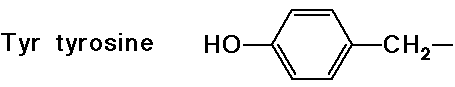
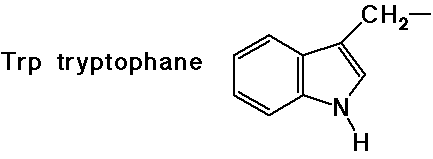
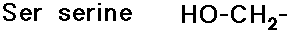

(also note that tyrosine is a phenol)
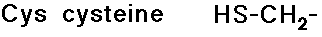
- carboxylic acid side chains

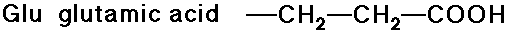
- amide side chains (from the above acids)

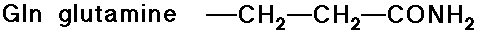
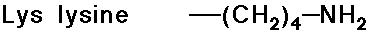
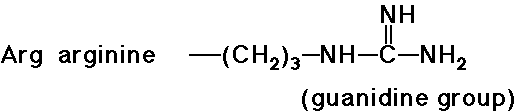
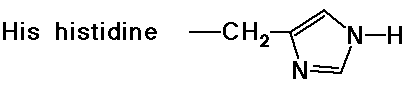
Acid-Base Properties of the Amino Acids
- basic amine group and acidic carboxyl group are typically both in their ionized forms in aqueous solution around biological pH values
- pKa for the alpha-amino group is about 9-10
- pKa for the carboxylic acid group is about 2-3
- below pH ~ 2, mainly cationic form (ammonium ion)
- between pH ~ 2 - 10, mainly zwitterionic form (both ammonium cation and carboxylate anion)
- above pH ~ 10, mainly anionic form (carboxylate anion)
- amino acids with side chains that can also ionize have more complicated behavior
The Isoelectric Point
- pH at which the major form of the molecule is neutral
(and there are equal - but small - amounts of cationic and anionic forms)
- for most amino acids, the isoelectric point is midway between their two pKa values (the middle of their neutral range)
- for amino acids with ionizable side chains, isoelectric points are at higher pH (for basic side chains) and lower pH (for acidic side chains)
Electrophoresis
- amino acids can be separated based on their charge in solution at a given pH
e.g., at pH 7, alanine is aprox. neutral, arginine is mainly +, glutamic acid is mainly -
- depending on their charge, the molecules migrate towards either a positive or negative electrode (moving through wet gel or paper)
Peptides - Amino Acids joined by Amide Bonds
- naming goes from N-terminus (free amine) to C-terminus (free carboxyl)
- -ine ending of the amino acids are replaced by -yl (except the last one)
e.g., threonylvaline is a dipeptide
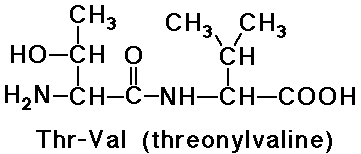
- note that valylthreonine would be a different dipeptide
Polypeptides
- peptides are abbreviated with 3-letter codes (sometimes 1-letter codes)
- note the huge variety of possible polypeptides, using just the 20 common amino acids as building blocks
20 amino acids
400 dipeptides
8,000 tripeptides
160,000 tetrapeptides
- typical proteins have 100 or more amino acids, so the variety is immense
- note also the variety of possible functional groups available in those 20 amino acids
- peptides have evolved with a complete array of properties useful for life processes
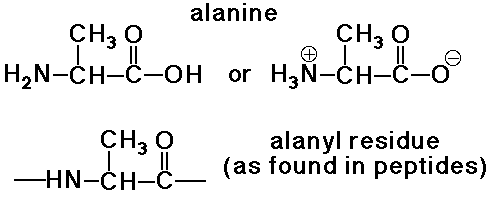
Amino Acid Residues
- an amino acid residue differs from an amino acid by H2O
Disulfide Linkages
- cysteine is readily crosslinked to another cysteine by a S-S bond

- disulfide linkages can hold together parts of a peptide chain that are not necessarily close in the peptide sequence
Amino Acid Analysis
- primary structure - the order of amino acids in the peptide chain
- complete hydrolysis (extended heating in aqueous HCl) breaks all amide bonds and releases all the individual amino acids
- individual amino acids can be separated by electrophoresis and/or chromatography and detected with ninhydrin (purple spot appears on reaction with any alpha-amino acid)
- complete hydrolysis, separation, and ninhydrin analysis can give a count of all amino acids and their relative abundance in the peptide
Partial Hydrolysis of Peptides
- occasionally, partial hydrolysis is carried out and smaller peptides are isolated, which are easier to analyze completely
- from the smaller peptides, sometimes useful connection information can be determined
- chymotrypsin and trypsin (digestive enzymes) are often used because they cleave peptides selectively
- chymotrypsin cleaves a peptide only on the carboxyl side of the aromatic amino acids (Phe, Tyr, Trp)
- trypsin cleaves a peptide only on the carboxyl side of the strongly basic amino acids (Lys and Arg)
Peptide Sequencing
- complete sequencing of a peptide can be done for up to about 20 amino acids in a row
- Edman degradation: selective cleavage of one amino acid off the N-terminus, followed by analysis to see what amino acid was removed
(the selective reagent is phenyl isothiocyanate, and the clipped-off product is a phenylthiohydantoin)
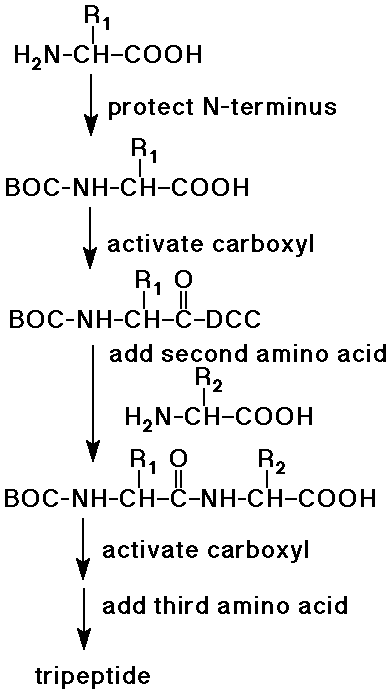
Peptide Synthesis
- complete synthesis of peptides was started by Emil Fischer
- the procedure now can be automated up to about 100 amino acids
- protecting groups are essential to control the coupling reactions
- protect the N-side of AA1
(e.g., as a BOC-amide - easily removed later)
- activate the C-side of AA1
(e.g., with DCC - milder than SOCl2)
- couple active AA1 to AA2
to form a dipeptide (AA2-AA1)
(note that the N-terminus is still protected)
- activate the free carboxyl group of the dipeptide, add AA3
(forms the tripeptide, AA1-AA2-AA3)
- repeat for each amino acid of the polypeptide
- eventually remove the protecting group from the N-terminus
Proteins - Natural Polypeptides
- protein classifications may be based on structure or function or origin
- proteins are always a polypeptide (sometimes more than one chain) and may also have other types of molecules associated
Conjugated Proteins are asociated with other types of molecules
- glycoproteins - sugars
- lipoproteins - lipids (nonpolar fats and oils)
- nucleoproteins - nucleic acids
- heme proteins - porphyrin molecule
- metalloproteins - metal ions (often in a heme group)
Protein Shapes
- fibrous proteins - side-by-side polypeptide chains
usually water-insoluble, mechanically strong
useful for structure, muscle
- globular proteins - compact, variable shapes
usually water-soluble, transported around the body
useful for specific functions like catalysis (enzymes)
Protein Functions
- enzymes - catalysts for controlling rates of specific reactions
- hormones - regulators of specific body processes
- transport - control of movement of other molecules or ions
- structure - muscle, skin, etc.
- storage - nutrient storage
- protection - antibodies
Protein Structure - Primary Structure
- amino acid sequence
- determination of the specific order of amino acid residues
Protein Secondary Structure
- regular conformations of the peptide chain
- alpha-helix - a coil held together by H-bonding
- beta-sheet (or pleated sheet) - antiparallel sections of peptide chains held together by H-bonding
Protein Tertiary Structure
- complete 3-dimensional structure of the protein
- includes disulfide bridges, ionic interactions, H-bonding, other polar interactions and nonpolar (hydrophobic) interactions
Protein Quaternary Structure
- aggregation of several peptides chains into a larger protein unit
Enzymes - Protein Catalysts
- enzymes affect the rate of a particular reaction
usually very specific as to what reaction or family of reactions will be catalyzed
- nomenclature: -ase suffix after a description of the reaction they catalyze
- hydrolase - hydrolysis
- isomerase - isomerization
- transferase - transfer of a group from one molecule to another
- lyase - elimination or addition of a small molecule, like water
- oxidoreductase - oxidation or reduction (usually with NADH or similar cofactor)
- ligase - binding of two molecules (usually with ATP cofactor)
- note that enzymes can catalyze a reaction in both directions
like any catalyst, they provide a lower-energy pathway between reactants and products
actual direction depends on the concentrations of substrates and cofactors
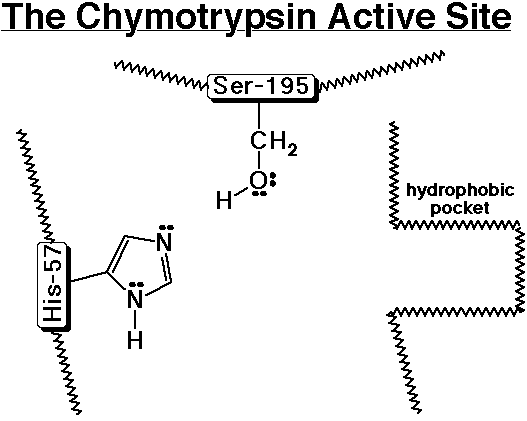
Chymotrypsin - A Specific Example of a Proteolytic Enzyme
- chymotrypsin cleaves an amide bond next to an aromatic amino acid
- the imidazole ring of a nearby histidine specifically adds or removes a proton to aid in nucleophilic attack or leaving group departure
Nucleic Acids
- huge molecules that carry the genetic information for an organism
- a polymer composed of phosphate esters linking sugars, with specific nitrogen bases attached to each sugar unit
Deoxyribose
- in RNA, ribose is the sugar, specifically beta-D-ribofuranose
- in DNA, 2-deoxyribose is the sugar, also with the beta furanose structure

The Nitrogen Bases
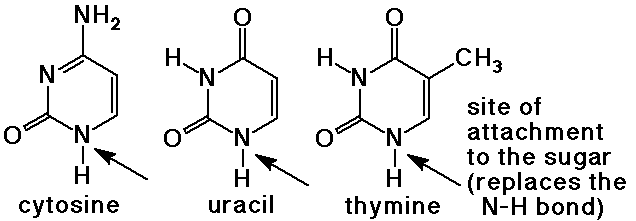
- pyrimidine bases have a 6-membered ring with two nitrogens
cytosine, uracil (found in RNA), and thymine (found in DNA)
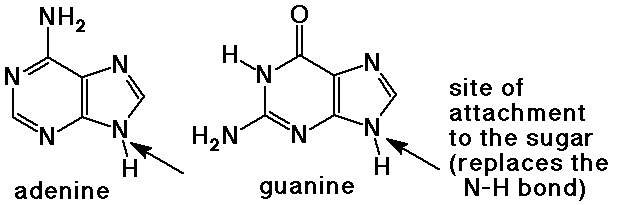
- purine bases have two rings with four nitrogen atoms
adenine and guanine
Nucleosides
- nitrogen base + sugar = nucleoside
- one-letter codes imply the nucleoside (add "d" for deoxy)
C = cytosine
U = uridine
T = thymidine
A = adenosine
G = guanosine
Nucleotides
- nitrogen base + sugar + phosphate(s) = nucleotide
- ATP = adenosine triphosphate is used as an energy storage molecule
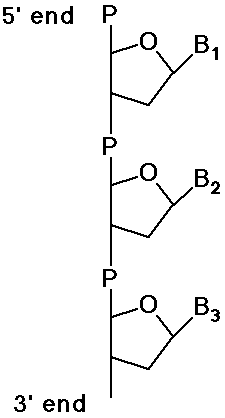
Polynucleotides - DNA and RNA
- polymers of DNA or RNA are named from 5' end to 3' end
Base Pairing
- specific H-bonding can take place between bases on adjacent strands
in DNA: A & T base pair, G & C base pair
in RNA: A & U base pair, G & C base pair
- the base pairing requires antiparallel directions of the two strands
the optimum structure for base pairing is a double helix
Replication of DNA
- note that each strand of DNA contains enough information to duplicate its partner
- DNA is replicated by uncoiling and creating new partners for each strand
- replication is done by an enzyme, DNA polymerase, using nucleotide triphosphates (like ATP)
Transcription of Genetic Information from DNA to RNA
- information from DNA is transcribed by copying onto messenger RNA (m-RNA)
- the m-RNA message matches the original DNA (complementary to the DNA being copied)
Translation of the Genetic Information into Protein Biosynthesis
- m-RNA brings the genetic information to ribosomes, where protein biosynthesis takes place
- m-RNA message is read sequentially by its 3-letter codons
- as needed, individual amino acids are brought to the ribosomes by transfer RNA (t-RNA)
- t-RNA specifically recognizes both the code for an amino acid and its particular amino acid
The Genetic Code
- each amino acid is represented by a three-letter code
the code is expressed as it appears on the m-RNA, from 5' to 3' direction
- special features of the genetic code (like a language)
- no punctuation - exact starting spot is crucial, must always move by 3 units
- there is a "stop" code
- duplicate codes - most amino acids have multiple codes
- single-letter changes (especially in the last spot) usually don't cause a serious misreading of the code
(however, sickle-cell anemia is caused by a single error in the structure of hemoglobin, in which 6-Glu is erroneously replaced by 6-Val)
Why is the OH Missing in DNA?
- RNA (both m-RNA and t-RNA) is meant to recycle readily to read and write messages
- DNA is meant to remain stable
- hydrolysis of the phosphate esters of nucleic acids is enhanced by the 2'-OH group
in the absence of the 2'-OH group, the phosphate esters are very stable to hydrolysis
DNA Sequencing
- sizes of DNA molecules measured in kilobases (1000s of base pairs)
- specific cleavages of DNA can occur with restriction enzymes
- smaller fragments are more easily identified and reassembled into the original
- the Human Genome Project is an attempt to map the entire human genetic information in DNA



![]()
![]()

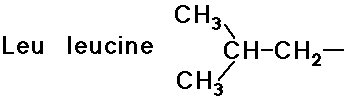
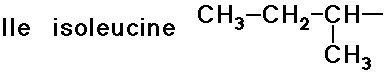


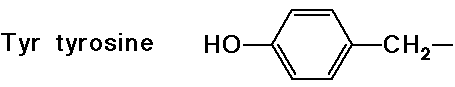

![]()

![]()
![]()
![]()
![]()
![]()
![]()
![]()
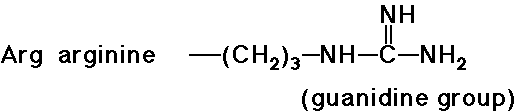
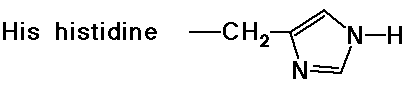
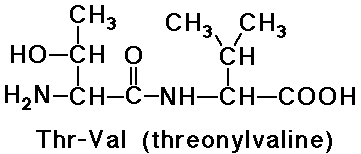

![]()




