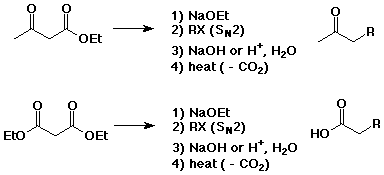Chapter 21 Notes - Ester Enolates

The Claisen Condensation
- condensation with a carboxyl derivative gives substitution
instead of addition
- the Claisen condensation gives beta-ketoesters
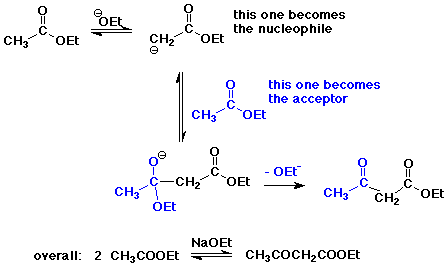
- crossed Claisen reaction - two different esters
works best of one has no alpha-hydrogens,
e.g., formate, carbonate, oxalate, or benzoate ester
- Dieckmann condensation - intramolecular Claisen - diester
gives cyclic product
Decarboxylation of beta-ketoacids
- Claisen products can be easily hydrolyzed and decarboxylated
Acetoacetic Ester and Malonic Ester Syntheses
- methods for preparing substituted acetones or acetic acids
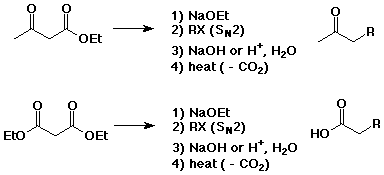
- if steps 1) and 2) are repeated, two substituents can be
attached
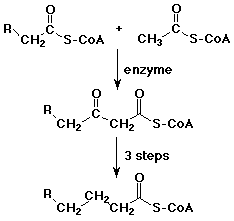
Biological Claisen Reactions
- acetyl Co-A often acts as a biological nucleophile at its
alpha-position
- formation of acetoacetyl Co-A is the first step in building
up fatty acids