Organic Chemistry III
Dr. Carl C. Wamser
1. (2 points) D-Threose is the aldotetrose shown below. Write it in an alpha-furanose form.
2. (3 points) The structure shown below is written in a
non-standard form.
Identify the anomeric carbon ( is it alpha or beta ? ).
Rewrite it in a standard Haworth projection.
Describe its structure relative to D-glucose.
The anomeric carbon is on the right in the standard Haworth projection. It is beta (up) in this compound.
The compound has opposite stereochemistry at C-2 and C-3 relative to D-glucose.
3. (2 points) Complete the reactions shown below. Write structures, not names, for the products.
a) D-glyceraldehyde + Tollen's reagent ---> 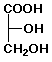
b) D- glucose + NaBH4 ---> 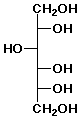
4. (3 points) Draw the structure of the disaccharide that
would result from joining D-allose (the C-3 epimer of D-glucose)
via its anomeric carbon in a beta form to the anomeric position
of D-glucose, also beta. Haworth projections are sufficient.
