Organic Chemistry III
Dr. Carl C. Wamser
1. (2 points each) Write the repeat unit of the polymer that would be formed from the following monomers:
a) 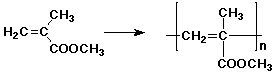
b) 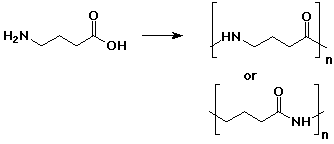
2. (3 points) Isoprene (shown below) forms natural rubber by a polymerization involving 1,4-additions. Write a repeat unit for natural rubber. Indicate what aspects of stereochemistry would need to be determined.
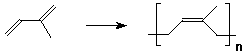
The product could be cis or trans. Natural rubber is actually cis.
3. (3 points) The copolymer shown below could be a substitute for polyethylene, with the property that it can be degraded by sunlight as indicated. Show the two different monomers that would be used in the synthesis of this copolymer.
Assuming the two monomers are equally reactive in the polymerization, what ratio of the two monomers would you use to synthesize the copolymer shown?
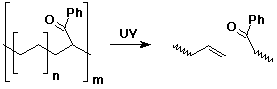
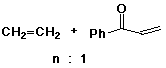
The molecular formula for the repeat unit implies n units of ethylene for every one unit of phenyl vinyl ketone.