Organic Chemistry III
Dr. Carl C. Wamser
1. (2 points) Give a systematic name for the following:
![]() N,N-diethyl-1-butanamine
N,N-diethyl-1-butanamine
or diethylbutylamine
2. (2 points) Draw a structure for N,N,N',N'-tetramethyl-1,4-butanediamine.
![]()
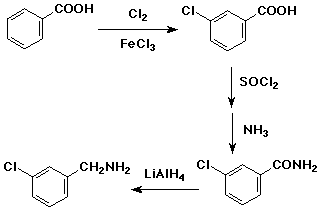
3. (3 points) Write a synthetic sequence that would convert benzoic acid to m-chlorobenzylamine. Show the reagents needed for each step, but mechanisms are not necessary.
4. (3 points) Write the steps needed and the products that would be formed during two stages of methylation / Hofmann elimination treatment of the compound below, resulting in ultimate elimination of trimethylamine.

(elimination in the opposite order is also possible, but the same product will result)