Organic Chemistry III
Dr. Carl C. Wamser
Brown & Foote, pages 1098 - 1104 :
Problems 27.10 - 15 , 17 - 20 , 23 - 32 , 34 - 37 , 39 - 46 ,
52 , 55 , 56
1. Write the structure of the tripeptide alanylprolyllysine, as it would appear at pH 7.
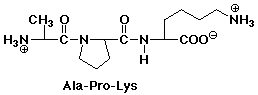
After acid hydrolysis into individual amino acids, show the products as they would appear at pH 1.
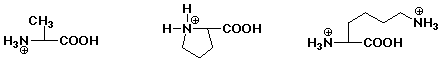
2. Show how you might start with a dipeptide, isoleucylglutamine, and specifically add a methionine to the N-terminus.
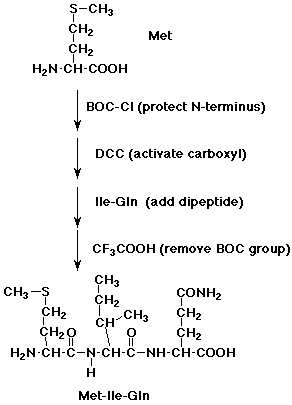
Repeat for the C-terminus.
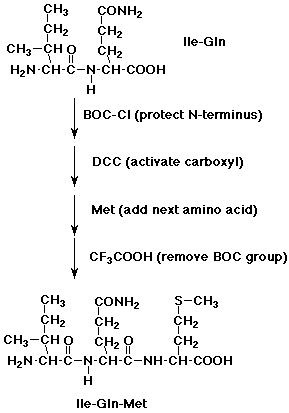
3. An unknown hexapeptide is determined by complete hydrolysis
to have the amino acid composition Ala (2), Lys, Glu, Phe, Cys.
Edman degradation releases Ala followed by Cys.
Chymotrypsin hydrolysis gives two tripeptides, both of which have
Ala at their N-termini.
Trypsin hydrolysis gives a pentapeptide plus Glu.
Identify the amino acid sequence of the hexapeptide.
Ala-Cys-Phe-Ala-Lys-Glu
4. A simple template for the active site of chymotrypsin is shown below. Chymotrypsin is a peptidase that specifically hydrolyzes a polypeptide next to (on the C-side) an aromatic amino acid (Phe, Tyr, or Trp). Use the template to follow the sequence of steps involved.
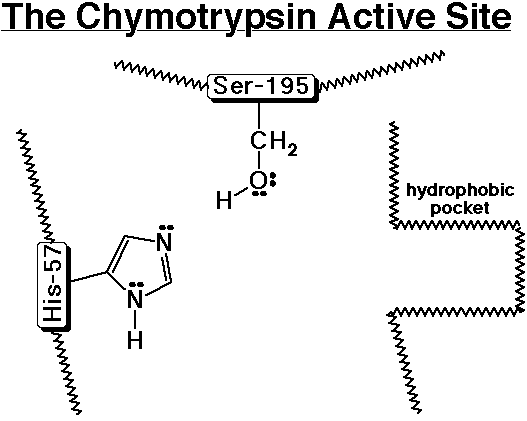
1) A polypeptide enters the active site and the aromatic amino acid side chain nestles into the hydrophobic pocket.
Nucleophilic substitution (2 steps)
2) Ser-195 acts as a nucleophile to add to the carbonyl group of the peptide, while His-57 removes a proton from Ser-195 to make it a better nucleophile.
3) The tetrahedral intermediate splits out an amino group, while His-57 donates a proton to the amino group to make it a better leaving group.
*******
4) The broken peptide fragment (the N-part) leaves the active site and is replaced by water.
Nucleophilic substitution (2 steps)
5) Water acts as a nucleophile to add to the carbonyl group of the intermediate (which is now an ester), while His-57 removes a proton from water to make it a better nucleophile.
6) The tetrahedral intermediate splits out the Ser-195 group, while His-57 donates a proton to Ser-195 to make it a better leaving group.
*******
7) The other part of the broken peptide (the C-part) leaves the active site and is replaced by another polypeptide (same as step 1 above).