Organic Chemistry III
Dr. Carl C. Wamser
Brown & Foote, pages 905 - 910 :
Problems 22.7 - 11 , 13 , 14 , 17 - 22 , 25 - 28 , 31 - 33
1. Write all the possible stereoisomers that could result from the Diels-Alder reaction of (E,E)-2,4-hexadiene with cis-2-butenedinitrile (cis-1,2-dicyanoethylene).
Given the preference for endo addition and minimizing steric hindrance, predict the major product(s). Would this product be described as racemic, achiral, or optically active?
**********
First assume that we are only considering stereoisomers in which the stereochemistry of the diene and the alkene are retained, as it is in a Diels-Alder reaction. Without this assumption, there are many other possibilities.
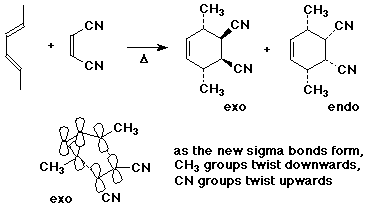
Because of the symmetry of these molecules, both the exo and endo products are meso compounds (achiral).