Organic Chemistry III
Dr. Carl C. Wamser
1. (15 points) Write clear structures for each of the following:
a) a D-ketotetrose

b) methyl b-D-glucopyranoside

c) poly(hexamethylene butanedioate)
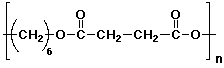
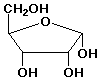
d) a-D-ribofuranose
e) the HOMO of 1,3,5-hexatriene
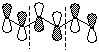
2. (15 points) Complete the following reactions:
a) 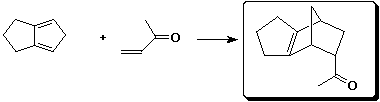
b) 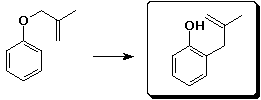
c) 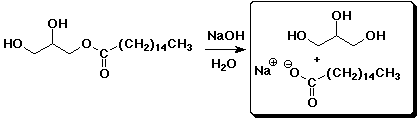
d) 
e) 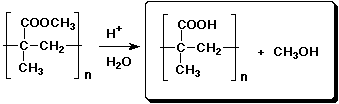
3. (15 points) Isoprene, shown below, could give four possible addition products with one equivalent of HBr. Identify the two most likely intermediates and the four possible products, allowing for both 1,2- and 1,4-additions. Identify the expected stereochemistry of all the products.

4. (15 points) D-Idose is shown below. Write the a-pyranose form in a Haworth projection and in both possible chair conformations. Predict the more stable conformation.
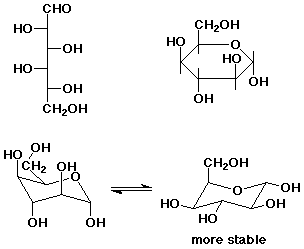
5. (15 points) Leukotriene C4 has the structure shown
below.
Identify (circle and name them) all of the different functional
groups present (there are at least ten).
Identify each stereocenter as R or S and each double bond as E
or Z, where appropriate.
Count the number of carbons in the main chain and predict what
family of compounds leukotriene is related to.
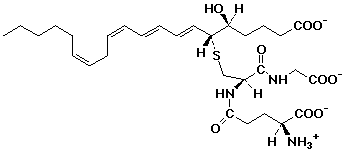
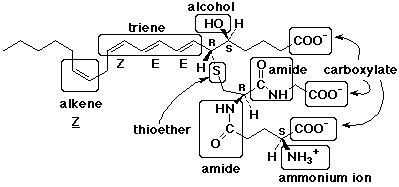
There are 20 carbons in the main chain derived from a fatty acid. Most likely this is from arachidonic acid, like the prostaglandins.
6. (10 points) Poly(methyl methacrylate) or PMMA, shown below, is usually created by anionic addition polymerization because the intermediate anion on the growing chain is well-stabilized. Use a general anion initiator Z- to start the polymerization and show one additional step of the polymerization. Illustrate how the intermediate anion is stabilized.
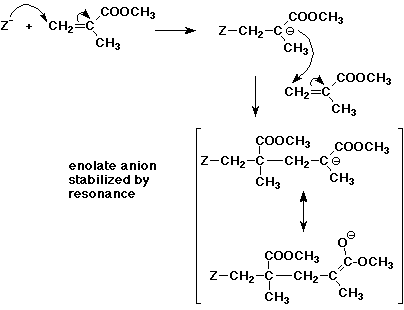
7. (15 points) Write a complete mechanism that shows the
mutarotation of D-glucose in aqueous acidic solution. Show all
steps and all resonance forms for any intermediates involved.
Include in your mechanism a pathway to form open-chain D-glucose.

Note that mutarotation could also be accomplished
by protonation of the anomeric -OH, followed by loss of H2O, then readdition of H2O on
the opposite side (an SN1
mechanism). The ring would not open, however, and this mechanism
does not lead directly to the open-chain form.