Organic Chemistry III
Dr. Carl C. Wamser
1. (12 points) Write a complete name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 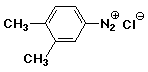
3,4-dimethylbenzenediazonium chloride
b) 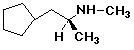
(S)-1-cyclopentyl-N-methyl-2-propanamine
c) 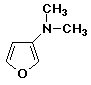
3-(dimethylamino)furan
2. (15 points) Arrange the following in order with respect to the property indicated. Just write "MOST" and "LEAST" under the compounds with the highest and lowest values of the property indicated.
a) basicity

MIDDLE / / MOST / / LEAST
b) basicity
![]()
MIDDLE / / MOST / / LEAST
c) reactivity towards a typical electrophile
![]()
MIDDLE / / LEAST / / MOST
d) reactivity towards a typical nucleophile
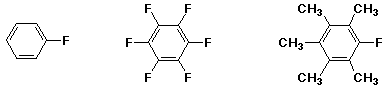
MIDDLE / / MOST / / LEAST
e) acidity
![]()
MIDDLE / / LEAST / / MOST
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 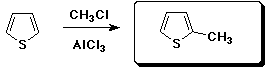
b) 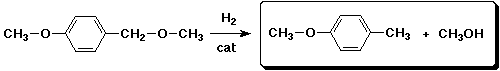
c) 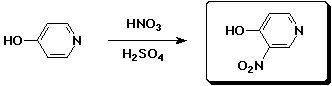
d) 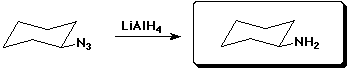
e) 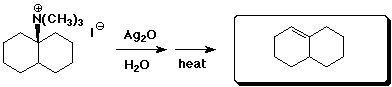
4. (16 points) Write a complete mechanism for the reaction
of chlorine with FeCl3 catalyst and p-dibromobenzene.
Show all steps and all resonance forms for any intermediates involved.

5. (15 points) Write a complete mechanism that explains the following reaction. Show all steps and all resonance forms for all intermediates.
If the alpha-carbon (the one originally attached to the
amine) was labeled with C-13, indicate at which position or positions
you would expect to find it in the product (use * to indicate
the labeled carbon).
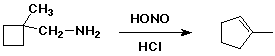
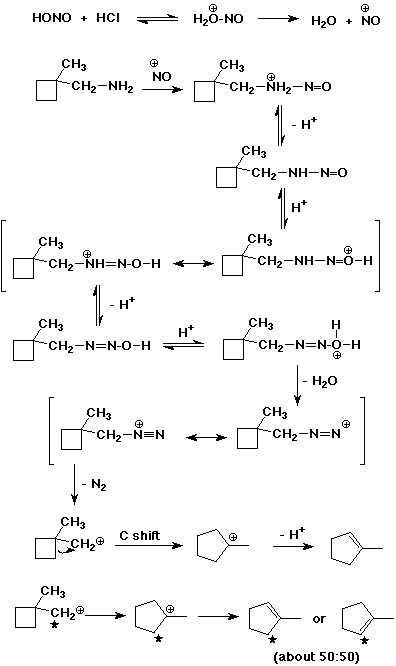
6. (12 points) Write a synthetic sequence to prepare the compound shown below, starting with aniline. Show the reagents and conditions needed for each separate step of the sequence.
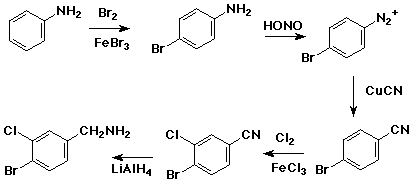
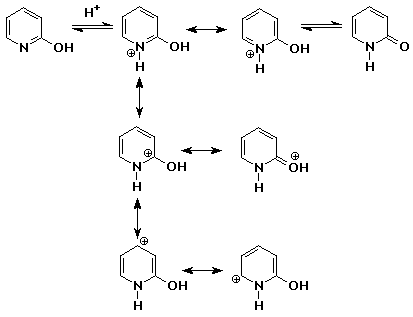
7. (15 points) Pyridone, shown below, has two tautomeric forms that can be interconverted by either acid or base. Write the intermediate for each case with all resonance forms.
in base:
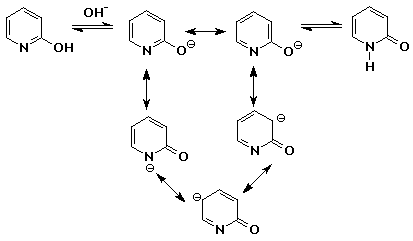
in acid: