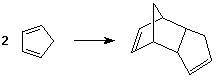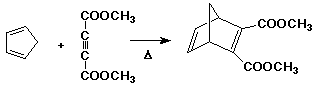Chapter 22 - Conjugated Dienes

Molecular Orbitals (MOs) of Conjugated Pi Systems
- n atomic orbitals (AOs) combine to make n molecular orbitals
(MOs)
- ethene - pi bond and pi* antibond, with two electrons in
the pi bond
- allyl pi system (could be cation, radical, or anion) - 3
pi MOs (bonding, nonbonding, antibonding)
- allyl cation (2 pi electrons), allyl radical (3 pi electrons),
allyl anion (4 pi electrons)
- 1,3-butadiene - 4 pi MOs (two bonding and two antibonding)
with 4 pi electrons
- frontier MOs are the most important for electron pushing
- highest occupied MO - HOMO
- lowest unoccupied MO - LUMO
Conjugate Addition to Dienes
- conjugated dienes are very reactive towards electrophiles
- addition gives a relatively stable allylic cation
- conjugated dienes can give 1,2 or 1,4 addition
- the two ends of the allyl cation may be nonequivalent
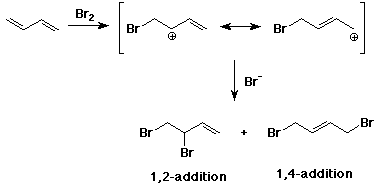
- addition at low temperature favors the 1,2-addition product
(kinetically controlled)
- addition at high temperature favors the 1,4-addition product
(thermodynamically controlled)
- heating the kinetic mixture of products gives the thermodynamic
mixture (i.e., mostly 1,4)
- kinetic control (lower Ea) - the more substituted cation
has more + charge, reacts faster
- thermodynamic control - the more substituted alkene is the
more stable product
- thermodynamic control requires equilibration to form the
more stable product, since it has a higher barrier
- Note - this situation of the more stable product having a
higher activation barrier is unusual
- in most situations, a more stable product means a lower barrier
(The Hammond Postulate)
The Diels-Alder Reaction
- cycloaddition of a diene to an alkene (usually called a dienophile
in this reaction)
- overall, two pi bonds are converted to two sigma bonds while
making the ring
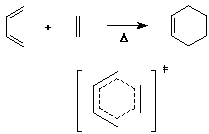
- note that the transition state is a 6-membered ring of delocalized
pi electrons
- such transition states are particularly stable when they
have (4n+2) electrons
- the diene must be in an s-cis conformation for this reaction
- s-cis means the single bond between the two double bonds
is cis
- cyclic dienes are much more reactive than acyclic dienes
- cyclopentadiene undergoes reaction with itself
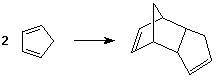
- the reaction goes best with alkenes having electron-withdrawing
substituents (dienophiles)
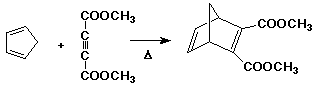
- this allows good electron flow from the diene HOMO to the
alkene LUMO
- Lewis acids also enhance the Diels-Alder reaction by complexing
the dienophile
- the combination of electron-deficient diene with electron-rich
alkene also works
- stereochemistry of the diene and the alkene is retained
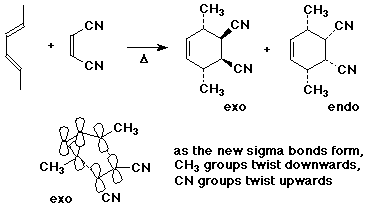
- endo product is usually the major product, due to secondary
interactions between the pi systems
Other Pericyclic Reactions
- pericyclic reactions have cyclic delocalized transition states
and are more stable when the transition stste unvolves (4n+2)
electrons (aromatic)
- cycloadditions (e.g., the Diels-Alder reaction) are just
one type of reaction termed "pericyclic"
- cycloadditions make two new sigma bonds in a new ring
- electrocyclic reactions make one new sigma bond in a new
ring, by joining the ends of a pi system
- sigmatropic reactions shift the position of a sigma bond
with respect to a pi system
- Claisen rearrangement and Cope rearrangement are examples
- Cope rearrangement
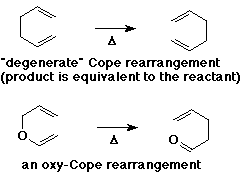
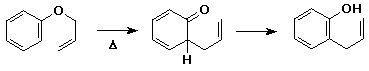
- Claisen rearrangement - special case of an oxy-Cope rearrangement
- allyl phenyl ether -> o-allylphenol