1. (2 points each) Attached are IR spectra for compounds X and Y, taken from the following list of possibilities:
cyclohexanone , t-butyl methyl ether , 2-methyl-1-butanol , 1-hexyne .
Identify which compound would give each spectrum and point out at least one feature in each spectrum that leads you to that conclusion.
X is t-butyl methyl ether
no O-H, no double or triple bond
Y is 2-methyl-1-butanol
strong O-H stretching peak
2. (2 points each) Give a specific example of a peak in either the IR or the UV that could be used to distinguish between the following pairs of compounds:
a) benzene and cyclohexanol
UV: only benzene will absorb
IR: bezene will show C=C, cyclohexanol will show O-H
b) 1,3-pentadiene and pentane
UV: only 1,3-pentadiene will absorb
IR: only 1,3-pentadiene will show C=C and sp2 C-H
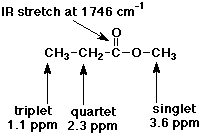
3. (2 points) Identify compound Z ( C4
H8 O2 ) from its
IR and NMR spectra.