1. (6 points) Describe the expected proton NMR spectra of the following compounds, including for each signal in the spectrum the chemical shift, relative intensity or number of hydrogens, and splitting pattern, using the general format shown in question 2.
a) methyl isopropyl ether
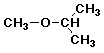
3.3 - 4.0 ppm, 3H, singlet
3.3 - 4.0 ppm, 1H, septet
0.9 - 2.0 ppm, 6H, doublet
b) 2-methyl-2-pentene
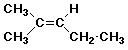
1.6 - 2.6 ppm, 3H, singlet
1.6 - 2.6 ppm, 3H, singlet
1.6 - 2.6 ppm, 2H, multiplet (or quartet of doublets)
5.0 - 5.7 ppm, 1H, triplet
0.9 - 2.0 ppm, 3H, triplet
2. (4 points) Identify the unknown compound of formula C5H12O2 that gives the proton NMR spectrum described below. Assign each of the signals to specific hydrogens in the compound.
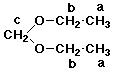
a -- 1.7 ppm, 6H, triplet
b -- 3.4 ppm, 4H, quartet
c -- 4.9 ppm, 2H, singlet