1. (12 points) Write a complete name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 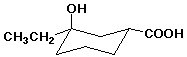
b) 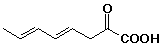
c) 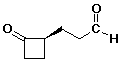
2. (15 points) Write accurate structures that illustrate each of the following:
a) trans-1,4-cyclohexanedicarboxylic acid
b) (R)-3-methyloctan-2,7-dione
c) disodium oxalate
d) the hydrazone of benzaldehyde
e) the products of a McLafferty rearrangement of octanoic acid
3. (15 points) Complete each of the following reactions
by adding the missing part:
either the starting compound, the necessary reagents and conditions,
or the final major product.
Indicate stereochemistry if it is specific.
a) 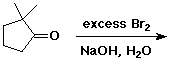
b) 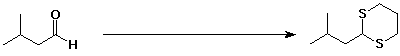
c) 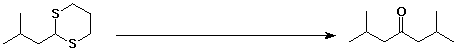
d) 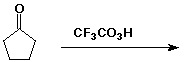
e) 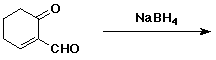
4. (12 points) Write a complete mechanism for the addition of ethylene glycol to acetone to make a cyclic ketal. Show all steps and all intermediates involved.
5. (10 points) Write a complete mechanism for the addition of methylmagnesium bromide to cyclopentanecarboxaldehyde. Include the usual acid workup step, using HCl as the acid. Show all steps and write every step as a balanced reaction.
6. (12 points) Identify the unknown compound S from the following spectra. For each spectrum give at least three specific assignments that help you identify the structure.
7. (10 points) Write a mechanism for the following reaction, and explain how it relates to the Wittig reaction.
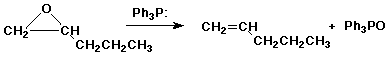
8. (14 points) Write out a sequence of reactions that could be used to accomplish EITHER ONE of the following conversions. Show the necessary reagents and conditions for each reaction step, but do not show mechanisms. As starting materials you may use acetylene, any alcohol that has three carbons or fewer, plus any needed solvents or inorganic reagents. JUST DO ONE OF THE TWO.